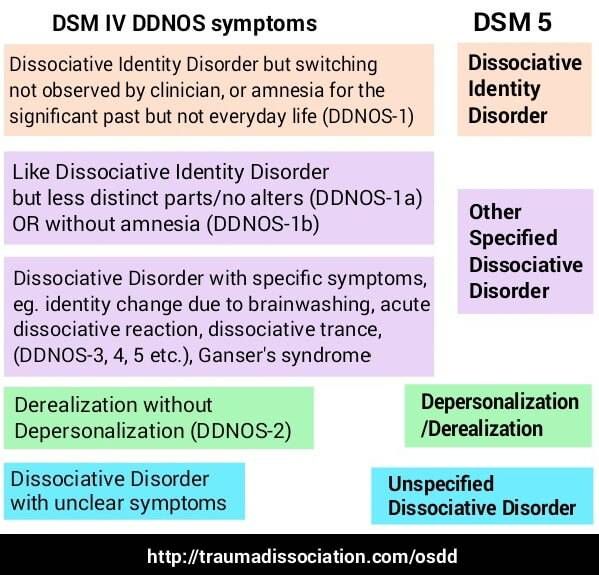
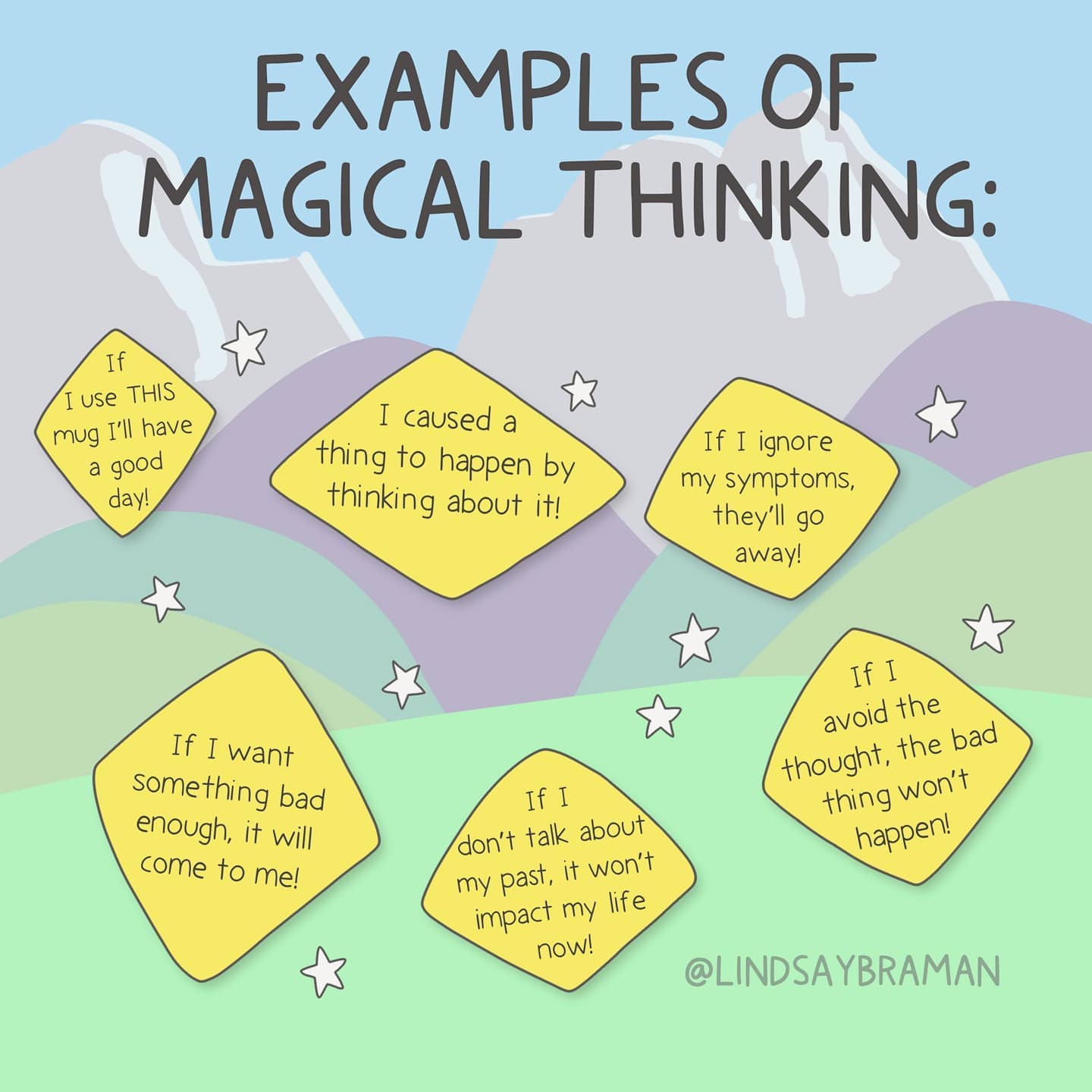
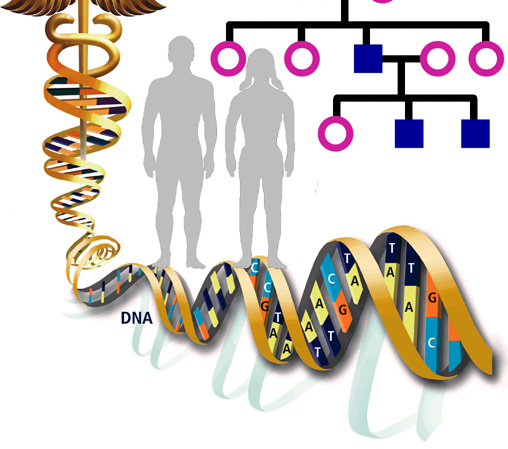
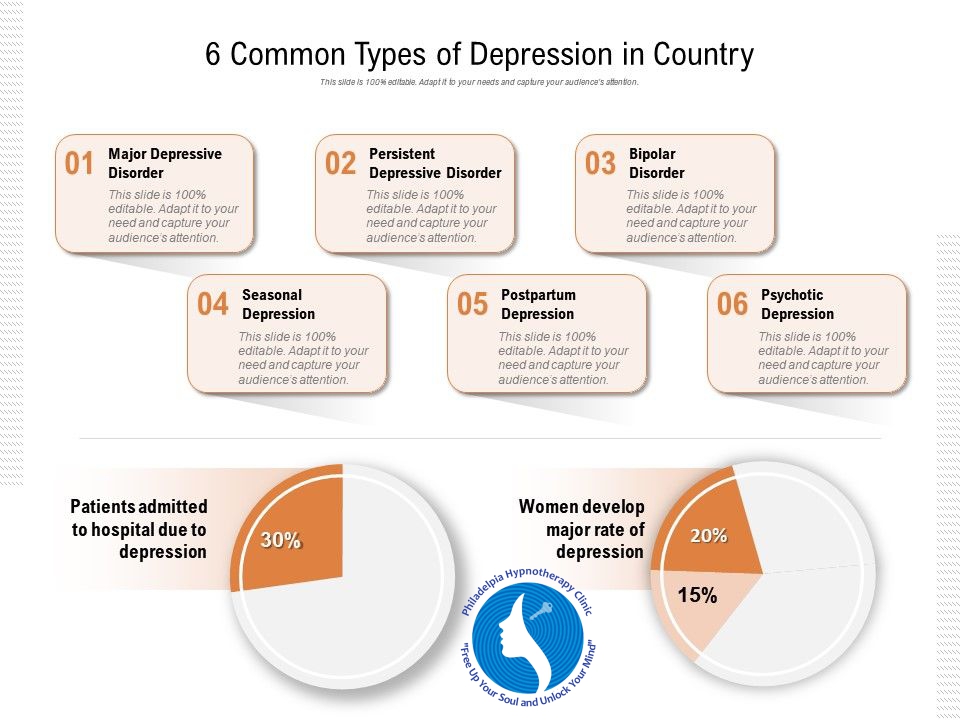
Genetic predisposition for schizophrenia
Causes - Schizophrenia - NHS
The exact causes of schizophrenia are unknown. Research suggests a combination of physical, genetic, psychological and environmental factors can make a person more likely to develop the condition.
Some people may be prone to schizophrenia, and a stressful or emotional life event might trigger a psychotic episode. However, it's not known why some people develop symptoms while others do not.
Increased risk
Genetics
Schizophrenia tends to run in families, but no single gene is thought to be responsible.
It's more likely that different combinations of genes make people more vulnerable to the condition. However, having these genes does not necessarily mean you'll develop schizophrenia.
Evidence that the disorder is partly inherited comes from studies of twins. Identical twins share the same genes.
In identical twins, if a twin develops schizophrenia, the other twin has a 1 in 2 chance of developing it, too. This is true even if they're raised separately.
In non-identical twins, who have different genetic make-ups, when a twin develops schizophrenia, the other only has a 1 in 8 chance of developing the condition.
While this is higher than in the general population, where the chance is about 1 in 100, it suggests genes are not the only factor influencing the development of schizophrenia.
Brain development
Studies of people with schizophrenia have shown there are subtle differences in the structure of their brains.
These changes are not seen in everyone with schizophrenia and can occur in people who do not have a mental illness. But they suggest schizophrenia may partly be a disorder of the brain.
Neurotransmitters
Neurotransmitters are chemicals that carry messages between brain cells.
There's a connection between neurotransmitters and schizophrenia because drugs that alter the levels of neurotransmitters in the brain are known to relieve some of the symptoms of schizophrenia.
Research suggests schizophrenia may be caused by a change in the level of 2 neurotransmitters: dopamine and serotonin.
Some studies indicate an imbalance between the 2 may be the basis of the problem. Others have found a change in the body's sensitivity to the neurotransmitters is part of the cause of schizophrenia.
Pregnancy and birth complications
Research has shown people who develop schizophrenia are more likely to have experienced complications before and during their birth, such as:
- a low birthweight
- premature labour
- a lack of oxygen (asphyxia) during birth
It may be that these things have a subtle effect on brain development.
Triggers
Triggers are things that can cause schizophrenia to develop in people who are at risk.
These include:
Stress
The main psychological triggers of schizophrenia are stressful life events, such as:
- bereavement
- losing your job or home
- divorce
- the end of a relationship
- physical, sexual or emotional abuse
These kinds of experiences, although stressful, do not cause schizophrenia. However, they can trigger its development in someone already vulnerable to it.
Drug abuse
Drugs do not directly cause schizophrenia, but studies have shown drug misuse increases the risk of developing schizophrenia or a similar illness.
Certain drugs, particularly cannabis, cocaine, LSD or amphetamines, may trigger symptoms of schizophrenia in people who are susceptible.
Using amphetamines or cocaine can lead to psychosis, and can cause a relapse in people recovering from an earlier episode.
Research has shown that teenagers and young adults who use cannabis regularly are more likely to develop schizophrenia in later adulthood.
Want to know more?
- Mind: How can recreational drugs affect mental health?
- Mind: What causes schizophrenia?
Page last reviewed: 11 November 2019
Next review due: 11 November 2022
Genetics of Schizophrenia - PMC
Curr Psychiatry Rep. Author manuscript; available in PMC 2018 Oct 17.
Published in final edited form as:
Curr Psychiatry Rep.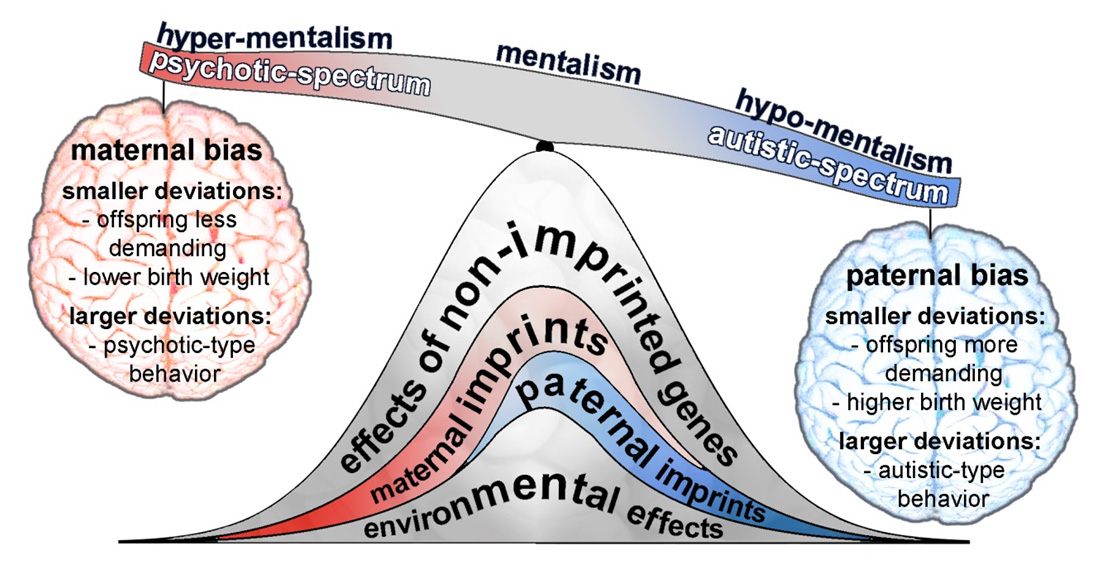 2014 Nov; 16(11): 502.
2014 Nov; 16(11): 502.
doi: 10.1007/s11920-014-0502-8
PMCID: PMC6192508
EMSID: EMS80014
PMID: 25200985
Author information Copyright and License information Disclaimer
The genetic basis of schizophrenia has been a hotly debated research topic for decades, yet recent studies, especially in the past year, have confirmed genetics as the major cause of this complex condition. Psychiatry has come of age: it is perhaps more difficult for the current generation of psychiatrists, to comprehend how the biological root of the condition could have been denied for so long. Here we review how highly collaborative global efforts to pool samples, utilise the very latest advances in genotyping and high throughput sequencing technologies, and application of robust statistical analysis have reaped phenomenal rewards. The major findings are that schizophrenia is a highly polygenic disorder with a complex array of risk loci, many include genes implicated also in intellectual disability, autism spectrum disorders, bipolar disorder and major depressive disorder.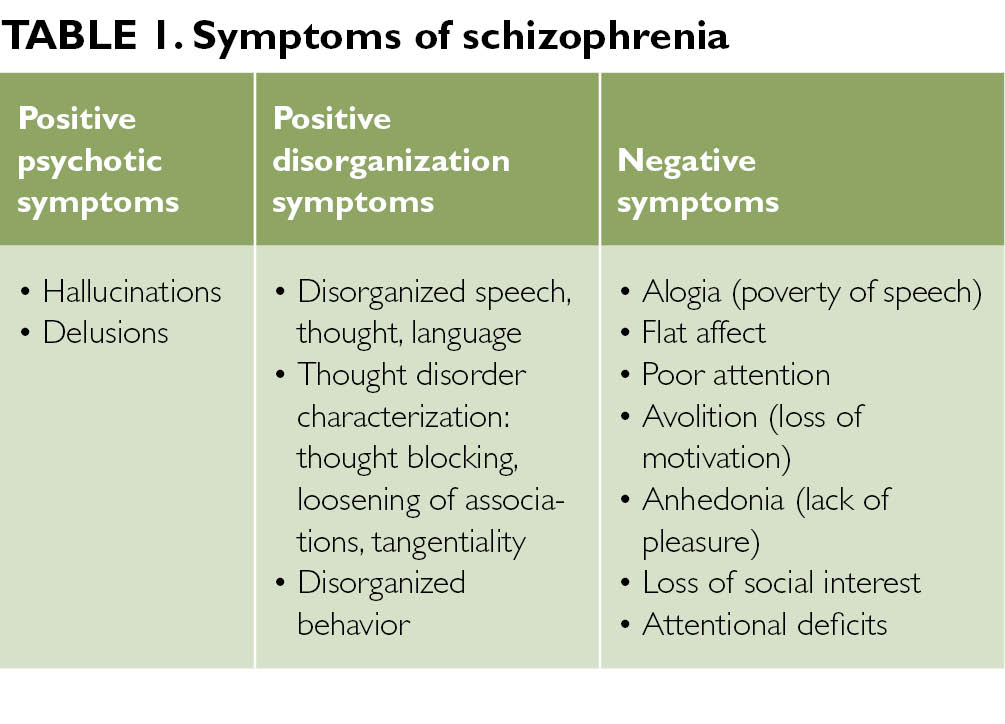 These candidate genes converge on key neuronal signalling pathways identifying novel targets for potential future therapeutic intervention.
These candidate genes converge on key neuronal signalling pathways identifying novel targets for potential future therapeutic intervention.
Keywords: Schizophrenia, Genetics, Copy number variants, Single nucleotide polymorphisms, Common variants, Rare variants, Neurodevelopmental disorders
Schizophrenia is a severe psychiatric disorder affecting approximately 1 % of the population worldwide. Like many other common diseases, schizophrenia is complex and multifactorial, with contributions from multiple susceptibility genes, epigenetic, stochastic, and environmental factors [1]. The search for candidate genes has proven difficult and has been hampered by clinical and genetic heterogeneity. Family, twin and adoption studies have shown that genetic factors play a major role in the development of schizophrenia, which has an estimated heritability between 60-85 % [2], and a number of candidate risk genes have been shown to associate [3]. A neurodevelopmental model for schizophrenia has been postulated from epidemiological, developmental and neuroimaging studies where symptoms of psychosis are the end result of processes that started to go awry years before the illness onset [4, 5].
Schizophrenia has long been assumed to be a disorder of neuronal signalling: either dopamine- or glutamate-related [6]. The dopamine hypothesis of schizophrenia is based on the assumption that schizophrenia results from excess dopamine activity and drugs which increase dopamine activity (dopamine agonists such as amphetamines), can induce psychotic symptoms [7]. A second piece of evidence in support of dopamine, is that selective dopamine receptor antagonists are effective antipsychotics, and their efficacy is closely correlated with their ability to block dopamine D2-like receptors. This is the pharmacological basis of our current treatments for schizophrenia, which are partially effective but unfortunately none are disease-modifying. Glutamate dysfunction has also been implicated in schizophrenia. The glutamate hypothesis is based on the fact that antagonists of the N-methyl-D-aspartate receptors (NMDA-R) subtype of glutamate, such as phencyclidine (PCP) and ketamine cause behavioural and cognitive symptoms similar to the positive, negative and cognitive symptoms of schizophrenia when given to healthy individuals [8]. Increasingly, many genetic risk variants which associate with neurodevelopmental disorders such as schizophrenia, intellectual disability (ID), and autism spectrum disorders (ASDs) are being linked to glutamatergic neurotransmission pathways raising the potential for new glutamatergic compounds for the treatment of these disorders in future.
Increasingly, many genetic risk variants which associate with neurodevelopmental disorders such as schizophrenia, intellectual disability (ID), and autism spectrum disorders (ASDs) are being linked to glutamatergic neurotransmission pathways raising the potential for new glutamatergic compounds for the treatment of these disorders in future.
Two main hypotheses have been proposed to underlie the genetics of schizophrenia and include the ‘common disease-common variant’ (CD-CV) model and the ‘common disease-rare variant’ (CD-RV) model. The CD-CV model, based on large genome-wide association studies (GWAS) of hundreds of thousands of single nucleotide polymorphisms (SNPs), proposes that schizophrenia is caused by a large number of common variants that each in itself causes only a modest effect [9]. The CD-RV hypothesis however proposes that some rare, highly penetrant copy number variants (CNVs; segments of DNA with duplications and deletions) play an important role in schizophrenia susceptibility as shown by their increased frequency in disease from a number of genome-wide CNV studies and also alludes to a broader neuropsychiatric spectrum for schizophrenia than previously conceived [10]. Furthermore, it also opens the door in future to the possibility of clinical diagnostic subtyping of schizophrenia based on genotype as well as phenotype data. In this review, we have presented the latest genomic findings in schizophrenia research and consider their implications to our current understanding of the condition.
Furthermore, it also opens the door in future to the possibility of clinical diagnostic subtyping of schizophrenia based on genotype as well as phenotype data. In this review, we have presented the latest genomic findings in schizophrenia research and consider their implications to our current understanding of the condition.
GWAS are based on the identification of multiple SNPs, both in cases and controls for a specific trait, in order to assess whether an allelic variant is more prevalent in cases compared to controls. Once this association has been found, the genes that co-segregate with this specific SNP will be putatively associated with the trait. These studies require a large number of participants in order to achieve enough statistical power to find variants with low relative risks with whole-genome significance, and this is thought to be the reason why earlier GWAS in psychiatry failed to find robust and replicable gene candidates. Multiple genes have since been found in schizophrenia GWAS, supporting the CD-CV model. In this model common genetic variants, present in the general population, would account for only a small contribution towards the risk of developing the disease. Although each allele has a low odds ratio (usually around 1.2), the totality of the genes could amount as much as 32-36 % of the genetic variance of the condition [11]. However, one criticism of GWAS has been population stratification. This concept refers to the possibility of obtaining spurious results due to allelic ancestral differences between the subpopulations in the study, rather than those associated to the studied trait [12]. Another problem may arise from underpowered studies that give rise to false negatives and the elimination of mitochondrial DNA and sex chromosomes from the studies could also prevent finding some associations [12].
In this model common genetic variants, present in the general population, would account for only a small contribution towards the risk of developing the disease. Although each allele has a low odds ratio (usually around 1.2), the totality of the genes could amount as much as 32-36 % of the genetic variance of the condition [11]. However, one criticism of GWAS has been population stratification. This concept refers to the possibility of obtaining spurious results due to allelic ancestral differences between the subpopulations in the study, rather than those associated to the studied trait [12]. Another problem may arise from underpowered studies that give rise to false negatives and the elimination of mitochondrial DNA and sex chromosomes from the studies could also prevent finding some associations [12].
The need for extensive samples in order to detect these variants led to the establishment of different consortia, including the International Schizophrenia Consortium (ISC), Molecular Genetics of Schizophrenia (MGS), SGENE and the Schizophrenia Working Group of the Psychiatric Genomics Consortium (SWG of PGC). The first meaningful result came from a combined sample between the ISC, SGENE and MGS and identified the major histocompatibility complex (MHC) as a risk factor (with a genome-wide P value of 9.5x10-9 for rs13194053, an SNP in the MHC region) [11]. Further studies showed association of schizophrenia with new loci: 1p21.3 (MIR137), 2q32.3 (PCGEM1), 8p23.2 (CSMD1), 8q21.3 (MMP16) and 10q24.32-33 [13]. Another 13 regions were described in a combined sample of PGC and Swedish participants, amongst them CACNB2, MAD1L1 and lincRNAs [14].
The first meaningful result came from a combined sample between the ISC, SGENE and MGS and identified the major histocompatibility complex (MHC) as a risk factor (with a genome-wide P value of 9.5x10-9 for rs13194053, an SNP in the MHC region) [11]. Further studies showed association of schizophrenia with new loci: 1p21.3 (MIR137), 2q32.3 (PCGEM1), 8p23.2 (CSMD1), 8q21.3 (MMP16) and 10q24.32-33 [13]. Another 13 regions were described in a combined sample of PGC and Swedish participants, amongst them CACNB2, MAD1L1 and lincRNAs [14].
It is worth highlighting that MHC is the most extensively associated gene for schizophrenia in GWAS: a locus on chromosome 6, that includes MHC, is by far the most significant association found to date. An immune factor in the disease can be implicated from the fact that maternal infection has been proposed as one of the factors increasing schizophrenia risk, which could account for as much as one-third of cases [15].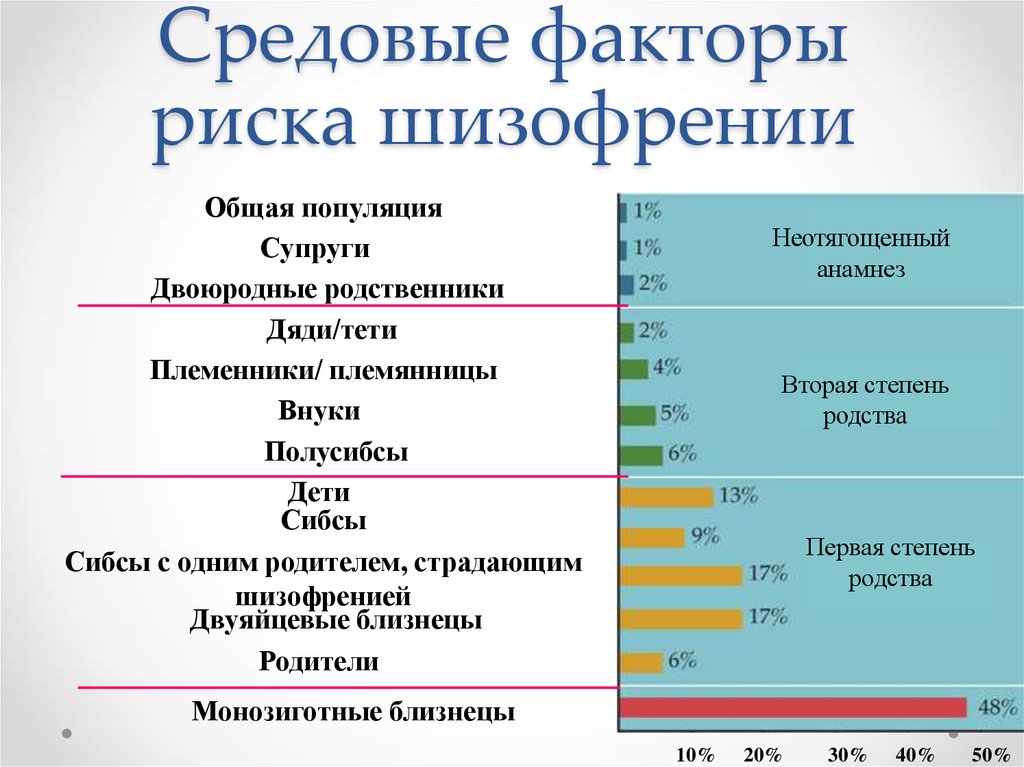 Moreover, studies have shown that offspring of mice injected with poly(I:C), a dsRNA that mimics viral infection, present alterations that resemble some schizophrenia features (e.g. enlarged ventricular volume, behavioural abnormalities) [16]. Further support for the role of MHC in schizophrenia comes from the fact that the molecule has also been found on synaptosomes in the adult mammalian CNS, in visual cortex synapses [17] and in neurons and neuronal precursor cells in the prenatal mouse brain [18]. In cultured neurons before and during the peak of synaptogenesis, MHC-I negatively correlates with glutamatergic synapses, being MHC more present in proximal dendrites and glutamatergic synapses in distal dendrites [17]. This inverse correlation was further observed in vivo, with an increase of more than 50 % in synaptic density in ‘knock-out’ MHC-I mice [17]. The implication for MHC-I, seen in glutamatergic synapses, is a strong argument for a possible role in the aetiology of schizophrenia, given the alterations in glutamate neurotransmission observed in patients [8].
Moreover, studies have shown that offspring of mice injected with poly(I:C), a dsRNA that mimics viral infection, present alterations that resemble some schizophrenia features (e.g. enlarged ventricular volume, behavioural abnormalities) [16]. Further support for the role of MHC in schizophrenia comes from the fact that the molecule has also been found on synaptosomes in the adult mammalian CNS, in visual cortex synapses [17] and in neurons and neuronal precursor cells in the prenatal mouse brain [18]. In cultured neurons before and during the peak of synaptogenesis, MHC-I negatively correlates with glutamatergic synapses, being MHC more present in proximal dendrites and glutamatergic synapses in distal dendrites [17]. This inverse correlation was further observed in vivo, with an increase of more than 50 % in synaptic density in ‘knock-out’ MHC-I mice [17]. The implication for MHC-I, seen in glutamatergic synapses, is a strong argument for a possible role in the aetiology of schizophrenia, given the alterations in glutamate neurotransmission observed in patients [8].
The immune system link has been further supported from recent biological insight reported in the findings of the SWG of PGC where by far the most significant locus includes a region containing the MHC [19••]. This latest paper comprised a multi-stage schizophrenia genome-wide association study of up to 36,989 cases and 113,075 controls and is the largest molecular genetic study of schizophrenia conducted to date [19••]. It identified associations spanning 108 conservatively defined loci of genome-wide significance (75 % protein coding genes; 40 % were a single gene and a further 8 % within 20 kb of a gene), 83 which had never been reported previously. A number of interesting associations were noted as relevant to existing hypotheses of schizophrenia aetiology including Dopamine Receptor D2 (DRD2) the gene target of current antipsychotic medications used in clinical practice. Other candidates genes, involved in glutamatergic neurotransmission and synaptic plasticity (such as GRM3, GRIN2A, SRR, GRIA1), were also revealed as well as genes which encode voltage-gated calcium channel subunits (i. e. CACN A1C, CACNB2 and CACNA11), again extending earlier findings of an overlap between genes affected by rare variants and those within GWAS loci, which were speculated to have a putative role in schizophrenia by resulting in abnormal glutamatergic synaptic and calcium channel functioning. Genes strongly expressed in cortical and striatal neuronal lineages were enriched for association also providing further evidence of a neuronal pathology in schizophrenia. Furthermore, closer scrutiny of the findings of this recent paper also reveals that the genetic effects in schizophrenia are enriched in regions outside the MHC that are also involved in acquired immunity [19••] providing further speculation to the longstanding theory that schizophrenia is a disorder of acquired immunity.
e. CACN A1C, CACNB2 and CACNA11), again extending earlier findings of an overlap between genes affected by rare variants and those within GWAS loci, which were speculated to have a putative role in schizophrenia by resulting in abnormal glutamatergic synaptic and calcium channel functioning. Genes strongly expressed in cortical and striatal neuronal lineages were enriched for association also providing further evidence of a neuronal pathology in schizophrenia. Furthermore, closer scrutiny of the findings of this recent paper also reveals that the genetic effects in schizophrenia are enriched in regions outside the MHC that are also involved in acquired immunity [19••] providing further speculation to the longstanding theory that schizophrenia is a disorder of acquired immunity.
Before this recent landmark study from the SWG of PGC, other studies that had gone before it had adopted a different approach to analyse more samples. It has been estimated that around 15 % of cases initially diagnosed with bipolar disorder were later reclassified as schizophrenia and 4 % of schizophrenia cases were later reclassified as bipolar disorder, with only 47. 1 % of schizophrenia cases retaining this diagnosis over a 10-year period [20], which is thought to be due to a considerable overlap between the symptoms of these conditions. Moreover, coheritability of SNPs between disorders has shown a common genetic background between schizophrenia and bipolar disorder (genetic correlation coefficient, rg =0.68) and schizophrenia and major depressive disorder (rg=0.43) [21]. Therefore, some studies have pooled cases from different disorders in order to increase the statistical power of the study and identify a shared genetic risk. In a joint analysis between schizophrenia and bipolar disorder, three genes reached genome-wide significance: CACNA1C, ANK3 and ITIh4-ITIh5 [13]. Furthermore, a combined analysis of five psychiatric disorders (schizophrenia, bipolar disorder, major depression, autism spectrum disorder and attention-deficit hyperactivity disorder) found three loci associated with the totality of the disorders (ITIh4, AS3MT, CACNB2) and validated the previous finding of CACNA1C in schizophrenia and bipolar disorder [22].
1 % of schizophrenia cases retaining this diagnosis over a 10-year period [20], which is thought to be due to a considerable overlap between the symptoms of these conditions. Moreover, coheritability of SNPs between disorders has shown a common genetic background between schizophrenia and bipolar disorder (genetic correlation coefficient, rg =0.68) and schizophrenia and major depressive disorder (rg=0.43) [21]. Therefore, some studies have pooled cases from different disorders in order to increase the statistical power of the study and identify a shared genetic risk. In a joint analysis between schizophrenia and bipolar disorder, three genes reached genome-wide significance: CACNA1C, ANK3 and ITIh4-ITIh5 [13]. Furthermore, a combined analysis of five psychiatric disorders (schizophrenia, bipolar disorder, major depression, autism spectrum disorder and attention-deficit hyperactivity disorder) found three loci associated with the totality of the disorders (ITIh4, AS3MT, CACNB2) and validated the previous finding of CACNA1C in schizophrenia and bipolar disorder [22]. Another relevant finding is MIR137, which is a microRNA implicated in neurogenesis and neuronal maturation and has CACNA1C and other schizophrenia risk genes as targets [13]. Mir-137 risk variant rs1625579 is associated with prefrontal cortex hyperactivity together with baseline levels of memory and cognition, which has been noted as neuronal inefficiency caused by mir-137 intervention in abnormal connectivity [23]. Furthermore, overexpression of mir-137 downregulates a subset of genes under the category of ‘regulation of neuronal differentiation’ [24] and high levels of mir-137 increase neuronal proliferation and inhibit neuronal differentiation in an in vivo assay [25].
Another relevant finding is MIR137, which is a microRNA implicated in neurogenesis and neuronal maturation and has CACNA1C and other schizophrenia risk genes as targets [13]. Mir-137 risk variant rs1625579 is associated with prefrontal cortex hyperactivity together with baseline levels of memory and cognition, which has been noted as neuronal inefficiency caused by mir-137 intervention in abnormal connectivity [23]. Furthermore, overexpression of mir-137 downregulates a subset of genes under the category of ‘regulation of neuronal differentiation’ [24] and high levels of mir-137 increase neuronal proliferation and inhibit neuronal differentiation in an in vivo assay [25].
A problem that is apparent from the aforementioned studies in the CD-CV model is the missing heritability. Since all the variants, in the large-scale studies conducted thus far, cannot entirely explain the heritability values set for schizophrenia, there has been a need for a complementary approach to potentially explain this discrepancy. In the CD-RV model the disorder is proposed to be caused by mutations in a single gene, which would localize to a diverse subset of genes. The main criticism to the CD-RV model however, is that rare, highly penetrant mutations would be negatively selected [26].
In the CD-RV model the disorder is proposed to be caused by mutations in a single gene, which would localize to a diverse subset of genes. The main criticism to the CD-RV model however, is that rare, highly penetrant mutations would be negatively selected [26].
One of the first single gene studies supporting this model found an association of a translocation between chromosome 1 and chromosome 11 with several mental disorders in a large Scottish pedigree [27, 28]. This translocation disrupted a gene in chromosome 1, which was accordingly named disrupted-in-schizophrenia-1 (DISC1), and a non-coding RNA, DISC2, which is transcribed antisense from DISC1. A study in a large cohort of 1542 cases (schizophrenia, bipolar disorder and recurrent major depressive disorder) and controls detected 905 SNPs in DISC1 with more than 1 % MAF (minor-allele frequency) and 3777 SNPs with less than 1 % MAF (‘rare’), of which 40 % were previously unknown. Amongst the rare variants, a R37W mutation that falls into a nuclear localization sequence was highlighted since it is algorithmically predicted to have a functional impact [29]. Further studies of DISC1 have shown its role as a scaffolding protein with a prominent function in brain development, neurogenesis and neuronal migration and in synaptic function (DISC1 interactome has been found to be enriched in synaptic proteins and proteins associated with NMDAR signalling). A role in oligodendrocyte development has also been suggested [30]. A role for DISC1 in mitochondrial trafficking has been proposed, through its interaction with Trak1, an interaction that is altered in R37W mutated DISC1. This is relevant due to the role mitochondria have in brain development and function as a response to changing energy needs [31]. Mouse models with mutated forms of DISC1 are also available and these have shown subtle brain alterations (reviewed in [32]).
Amongst the rare variants, a R37W mutation that falls into a nuclear localization sequence was highlighted since it is algorithmically predicted to have a functional impact [29]. Further studies of DISC1 have shown its role as a scaffolding protein with a prominent function in brain development, neurogenesis and neuronal migration and in synaptic function (DISC1 interactome has been found to be enriched in synaptic proteins and proteins associated with NMDAR signalling). A role in oligodendrocyte development has also been suggested [30]. A role for DISC1 in mitochondrial trafficking has been proposed, through its interaction with Trak1, an interaction that is altered in R37W mutated DISC1. This is relevant due to the role mitochondria have in brain development and function as a response to changing energy needs [31]. Mouse models with mutated forms of DISC1 are also available and these have shown subtle brain alterations (reviewed in [32]).
Recent exome-sequencing studies have also found evidence of an enrichment of rare variants in schizophrenia. These advances in gene sequencing allow us to identify rare pathogenic single base pair changes as well as insertion deletion polymorphisms. A polygenic burden of rare mutations, with minor allele frequencies of less than 0.5 % or singletons (alleles present in only one heterozygous individual), were significant in a Swedish sample of schizophrenia cases and controls [33•]. Moreover, these mutations were more prominent in the activity-regulated cytoskeleton-associated scaffold protein (ARC) of the post-synaptic density and post-synaptic density-95 (PSD-95) complexes and in genes related to calcium channels (such as CACNA1C) [33•]. In another important study which established an enrichment of de novo mutations in schizophrenia and linked them to specific biological pathways, an increased occurrence of mutations in genes previously implicated in the disease was found, especially in the NMDAR and ARC complexes and in Fragile X mental retardation protein (FMRP) targets [34••].
These advances in gene sequencing allow us to identify rare pathogenic single base pair changes as well as insertion deletion polymorphisms. A polygenic burden of rare mutations, with minor allele frequencies of less than 0.5 % or singletons (alleles present in only one heterozygous individual), were significant in a Swedish sample of schizophrenia cases and controls [33•]. Moreover, these mutations were more prominent in the activity-regulated cytoskeleton-associated scaffold protein (ARC) of the post-synaptic density and post-synaptic density-95 (PSD-95) complexes and in genes related to calcium channels (such as CACNA1C) [33•]. In another important study which established an enrichment of de novo mutations in schizophrenia and linked them to specific biological pathways, an increased occurrence of mutations in genes previously implicated in the disease was found, especially in the NMDAR and ARC complexes and in Fragile X mental retardation protein (FMRP) targets [34••].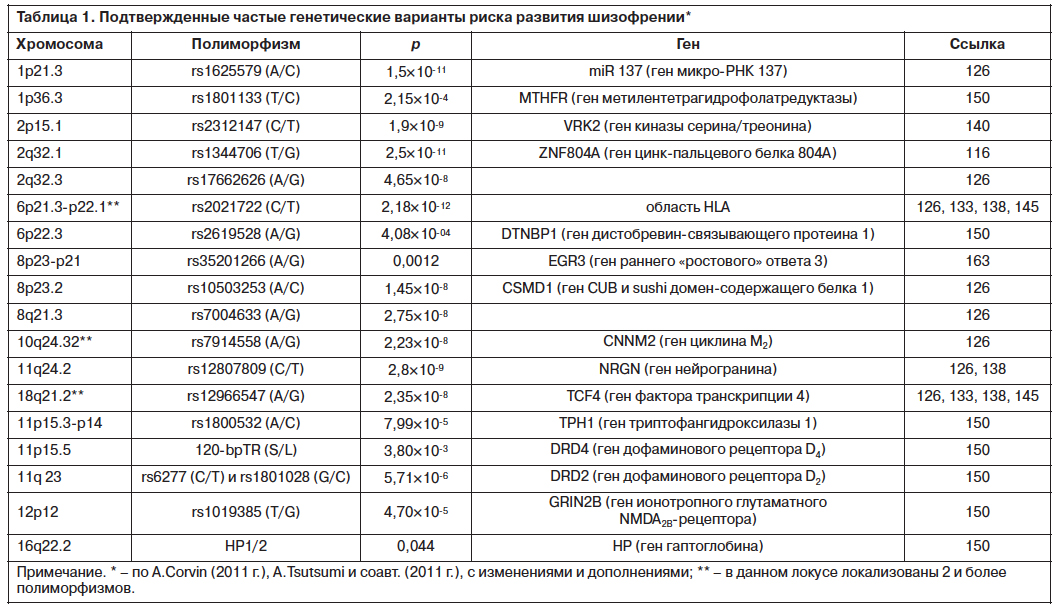 These results not only support the importance of rare mutations in schizophrenia, but also implicate specific pathways, such as calcium signalling and glutamate neurotransmission, that had been proposed already in GWAS.
These results not only support the importance of rare mutations in schizophrenia, but also implicate specific pathways, such as calcium signalling and glutamate neurotransmission, that had been proposed already in GWAS.
In addition to point mutations which have been discovered from recent sequencing studies, CNVs as mentioned earlier, have been found enriched in schizophrenia cases (1.15 case/control ratio)[35]. Duplications and/or deletions of numerous genomic regions have been implicated in the disease, and many of these include more than one gene (see for a list of some CNVs reported to associate with increased risk of schizophrenia). A relevant characteristic of CNVs in schizophrenia is the preponderance of de novo cases. It has been estimated that de novo CNVs are 8-fold more frequent in schizophrenia cases compared to controls, whereas sporadic schizophrenia cases are only 1.5-fold more likely to harbour inherited CNVs than controls [36]. Further analysis has shown an enrichment of de novo CNVs in synaptic genes, explained mostly by enrichment in the ARC complex and NMDAR genes [37••]. This is especially relevant, not only due to the pathophysiological links to the GWAS findings, but because this elevated de novo mutation rate has been proposed as a possible mechanism maintaining schizophrenia in the population, despite strong negative selection and the fact that the overall fertility of patients with schizophrenia, autism and anorexia nervosa is reduced [38].
This is especially relevant, not only due to the pathophysiological links to the GWAS findings, but because this elevated de novo mutation rate has been proposed as a possible mechanism maintaining schizophrenia in the population, despite strong negative selection and the fact that the overall fertility of patients with schizophrenia, autism and anorexia nervosa is reduced [38].
Table 1
List of CNVs associated with increased risk of schizophrenia [39]
| Region | Type of CNV | Region | Type of CNV |
|---|---|---|---|
| 1q21.1 | deletion/duplication | 15q11.2 | deletion/duplication |
| 2p16. | deletion/duplication | 15q13.3 | deletion/duplication |
| 3p26.1 | deletion/duplication | 16p11.2 | duplication |
| 3q29 | deletion | 16p13.1 | deletion/duplication |
| 5p13.2 | deletion/duplication | 17p12 | deletion |
| 7q11.2 | duplication | 17q12 | deletion/duplication |
| 7q22. | duplication | 22q11.2 | deletion |
| 7q36.3 | deletion/duplication | 15q13.1 | duplication |
Open in a separate window
Schizophrenia genetics is an example where persistence, collaboration, and utilisation of the latest technologies has been duly rewarded. Over the past few years there has been a flurry of important high impact papers from GWAS [14, 19], CNV [37••], and sequencing studies [33•, 34], which all suggest the importance of a number of genes involved in synaptic plasticity including ARC, NMDAR complexes, FMRP targets and voltage-gated calcium channels. The most recent of these studies was produced from a collaboration of over 80 research institutions world-wide, which comprise the SWG of PGC, and identified 108 genetic loci to schizophrenia, 83 of which are new [19••], and have brought renewed optimism to the field [40].
The extensive list of genes implicated in the pathophysiology of schizophrenia also brings it’s own challenges clinically. Pleiotropy refers to the genetic overlap between disorders, and it certainly appears that many of the conditions we diagnose in clinic including schizophrenia, affective disorders, ID, ASD, and attention-deficit hyperactivity disorder (ADHD) have shared genetic risk factors alluding to the possibility that they all share similar underlying disease pathways and cellular mechanisms so perhaps schizophrenia is best viewed as part of a spectrum of neurodevelopmental disorders. The concept of endophenotype may increasingly be adopted in psychiatric research where an endophenotype is defined as a measurable trait that is more related to the genetic underlying of a disease than clinical phenotype [41]. Different endophenotypes have been proposed, although one of the most cited is the P300 inhibition, which has been found associated with specific SNPs in mir-137, DISC1, ABCB1 and BDNF [42]. The development of a solid knowledge of endophenotypes and their genetic underpinnings may in future be helpful in redefining classification systems in psychiatric research given the large number of gene targets.
The fundamental goal of psychiatric genetics research is to achieve a greater understanding of the pathophysiological mechanisms underlying disease with a view to developing more tailored effective therapies in future. The studies discussed thus far have identified risk genes associated with schizophrenia but it is now essential to functionally validate these variants to shed light on the biological mechanisms underpinning the disease. An elegant study has recently been published using stem cells from patients with 15q11.2 CNVs [43••]. The authors took a multifaceted approach to investigate why 15q11.2 CNVs are prominent risk factors for schizophrenia and autism. Even in normal control subjects, carriers of the 15q11.2 deletion have cognitive deficits and structural changes on MRI scanning [44], which has raised interesting questions about how this genetic variant brings about the structural and functional changes seen in the carriers. They showed that human iPSC-derived neural progenitor cells carrying 15q11.2 microdeletions exhibited deficits in adherens junctions and apical polarity resulting from haploinsufficiency of CYFIP1 (a gene within 15q11.2 that encodes a subunit of the WAVE complex which regulates cytoskeletal dynamics) [43••]. Furthermore, they showed that deficiency in CYFIP1 and WAVE in the developing mouse cortex affects radial glial cell migration causing ectopic localisation outside of the ventricular zone. Therefore, by integrating human neural stem cells, in vivo animal modelling and targeted human genetic association studies they have provided a mechanistic understanding of how 15q11.2 microdeletions affect neural development. In future it will be possible to use such a multi-faceted biological approach to unravel the cellular architecture of complex neuropsychiatric disorders building on what we have learned from the genetic studies.
Irene Escudero is a Master’s student on exchange at the University of Edinburgh funded by a EUROlife Scholarship from the University of Barcelona, Spain. Mandy Johnstone is a PI at the CGEM and is supported by a Wellcome Trust Postdoctoral Clinical Fellowship. This work has also been funded by a starter grant from the Academy of Medical Sciences (to Mandy Johnstone) and a grant from the RS Macdonald Charitable Trust (to Mandy Johnstone).
Mandy Johnstone has received grants from the Wellcome Trust, RS Macdonald Charitable Trust, and the Academy of Medical Sciences.
Conflict of Interest Irene Escudero declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Irene Escudero, The Centre for Genomic and Experimental Medicine, The Institute of Genetics and Molecular Medicine, University of Edinburgh, Western General Hospital, Crewe Road, Edinburgh, Eh5 2XU, United Kingdom.
Mandy Johnstone, Division of Psychiatry, University of Edinburgh, Royal Edinburgh Hospital, Morningside Terrace, Edinburgh, Eh20 5HF, United Kingdom.
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
1. Ayhan Y, et al. Animal models of gene-environment interactions in schizophrenia. Behav Brain Res. 2009;204(2):274–81. [PMC free article] [PubMed] [Google Scholar]
2. Lichtenstein P, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–9. [PMC free article] [PubMed] [Google Scholar]
3. Rodriguez-Murillo L, Gogos JA, Karayiorgou M. The genetic architecture of schizophrenia: new mutations and emerging paradigms. Annu Rev Med. 2012;63:63–80. [PubMed] [Google Scholar]
4. Owen MJ, et al. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198(3):173–5. [PMC free article] [PubMed] [Google Scholar]
5. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17(12):1228–38. [PMC free article] [PubMed] [Google Scholar]
6. Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia–a synthesis and selective review. J Psychopharmacol. 2007;21(4):440–52. [PubMed] [Google Scholar]
7. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–9. [PubMed] [Google Scholar]
8. Umbricht D, et al. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57(12):1139–47. [PubMed] [Google Scholar]
9. Gershon ES, Alliey-Rodriguez N, Liu C. After GWAS: searching for genetic risk for schizophrenia and bipolar disorder. Am J Psychiatry. 2011;168(3):253–6. [PMC free article] [PubMed] [Google Scholar]
10. Bassett AS, Scherer SW, Brzustowicz LM. Copy number variations in schizophrenia: critical review and new perspectives on concepts of genetics and disease. Am J Psychiatry. 2010;167(8):899–914. [PMC free article] [PubMed] [Google Scholar]
11. Purcell SM, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–52. [PMC free article] [PubMed] [Google Scholar]
12. Bergen SE, Petryshen TL. Genome-wide association studies of schizophrenia: does bigger lead to better results? Curr Opin Psychiatry. 2012;25(2):76–82. [PMC free article] [PubMed] [Google Scholar]
13. Schizophrenia Psychiatric Genome-Wide Association Study. C. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–76. [PMC free article] [PubMed] [Google Scholar]
14. Ripke S, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45(10):1150–9. [PMC free article] [PubMed] [Google Scholar]
15. McAllister AK. Major histocompatibility complex I in brain development and schizophrenia. Biol Psychiatry. 2014;75(4):262–8. [PMC free article] [PubMed] [Google Scholar]
16. Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–21. [PubMed] [Google Scholar]
17. Glynn MW, et al. MHCI negatively regulates synapse density during the establishment of cortical connections. Nat Neurosci. 2011;14(4):442–51. [PMC free article] [PubMed] [Google Scholar]
18. Chacon MA, Boulanger LM. MHC class I protein is expressed by neurons and neural progenitors in mid-gestation mouse brain. Mol Cell Neurosci. 2013;52:117–27. [PubMed] [Google Scholar]
19. Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7.
[••This is a landmark multistage schizophrenia genome-wide association study of 36,989 cases and 113,075 controls. It identifies 128 independent associates spanning 108 conservatively defined loci that meet genome-wide significance of which 83 have never before been reported. Furthermore, these associates provide novel insight into the biological basis of schizophrenia] [PMC free article] [PubMed] [Google Scholar]
20. Bromet EJ, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168(11):1186–94. [PMC free article] [PubMed] [Google Scholar]
21. Cross-Disorder Group of the Psychiatric Genomics, C et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984–94. [PMC free article] [PubMed] [Google Scholar]
22. Cross-Disorder Group of the Psychiatric Genomics, C. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–9. [PMC free article] [PubMed] [Google Scholar]
23. van Erp TG, et al. Schizophrenia miR-137 locus risk genotype is associated with dorsolateral prefrontal cortex hyperactivation. Biol Psychiatry. 2014;75(5):398–405. [PMC free article] [PubMed] [Google Scholar]
24. Hill MJ, et al. Transcriptional consequences of schizophrenia candidate miR-137 manipulation in human neural progenitor cells. Schizophr Res. 2014;153(1–3):225–30. [PMC free article] [PubMed] [Google Scholar]
25. Szulwach KE, et al. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol. 2010;189(1):127–41. [PMC free article] [PubMed] [Google Scholar]
26. Mitchell KJ, Porteous DJ. Rethinking the genetic architecture of schizophrenia. Psychol Med. 2011;41(1):19–32. [PubMed] [Google Scholar]
27. Blackwood DH, et al. Schizophrenia and affective disorders–cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69(2):428–33. [PMC free article] [PubMed] [Google Scholar]
28. Millar JK, et al. Disruption of two novel genes by a translocation cosegregating with schizophrenia. Hum Mol Genet. 2000;9(9):1415–23. [PubMed] [Google Scholar]
29. Thomson PA, et al. 708 Common and 2010 rare DISC1 locus variants identified in 1542 subjects: analysis for association with psychiatric disorder and cognitive traits. Mol Psychiatry. 2014;19(6):668–75. [PMC free article] [PubMed] [Google Scholar]
30. Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12(12):707–22. [PMC free article] [PubMed] [Google Scholar]
31. Ogawa F, et al. DISC1 complexes with TRAK1 and Miro1 to modulate anterograde axonal mitochondrial trafficking. Hum Mol Genet. 2014;23(4):906–19. [PMC free article] [PubMed] [Google Scholar]
32. Johnstone M, et al. DISC1 in schizophrenia: genetic mouse models and human genomic imaging. Schizophr Bull. 2011;37(1):14–20. [PMC free article] [PubMed] [Google Scholar]
33. Purcell SM, et al. A polygenic burden of rare disruptivemutations in schizophrenia. Nature. 2014;506(7487):185–90.
[•This exome sequencing study of 2,536 schzophrenia cases and 2,543 controls demonstrated a polygenic burden from rare (less than 1 in 10, 000) disruptive mutations across many genes but with enrichment in genes associated with voltage-gated calcium channels, ARC and FMRP complexes. ] [PMC free article] [PubMed] [Google Scholar]
34. Fromer M, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506(7487):179–84.
[••This study shows that de novo CNVs occur in a small fraction of schizophrenia cases and disproportionately disrupt genes encoding post-synaptic density proteins including ARC and NMDAR complexes suggesting disruption of synaptic networks. Furthermore, mutations found in schizophrenia overlap with those found in autism and intellectual disability suggesting a shared genetic basis to a range of neurodevelopmental disorders.] [PMC free article] [PubMed] [Google Scholar]
35. ISC. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455(7210):237–41. [PMC free article] [PubMed] [Google Scholar]
36. Xu B, et al. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008;40(7):880–5. [PubMed] [Google Scholar]
37. Kirov G, et al. De novo CNVanalysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17(2):142–53.
[••This study of 662 schizophrenia proband-parent trios showed that rare de novo CNVs were significantly more frequent in cases compared to controls. Using a systems biology approach this study showed that de novo CNVs were significanly enriched for the post-synaptic density proteome (in partiular ARC and NMDAR complexes)] [PMC free article] [PubMed] [Google Scholar]
38. Power RA, et al. Fecundity of patients with schizophrenia, autism, bipolar disorder, depression, anorexia nervosa, or substance abuse vs their unaffected siblings. JAMA Psychiatry. 2013;70(1):22–30. [PubMed] [Google Scholar]
39. Doherty JL, O'Donovan MC, Owen MJ. Recent genomic advances in schizophrenia. Clin Genet. 2012;81(2):103–9. [PubMed] [Google Scholar]
40. Reardon S. Gene-hunt gain for mental health. Nature. 2014;511(7510):393. [PubMed] [Google Scholar]
41. Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37(2):163–80. [PMC free article] [PubMed] [Google Scholar]
42. Decoster J, et al. Genetic association study of the P300 endophenotype in schizophrenia. Schizophr Res. 2012;141(1):54–9. [PubMed] [Google Scholar]
43. Yoon KJ, et al. Modeling a Genetic Risk for Schizophrenia in iPSCs and Mice Reveals Neural Stem Cell Deficits Associated with Adherens Junctions and Polarity. Cell Stem Cell. 2014;15(1):79–91.
[••This study elegantly demonstrated how iPSC technology can be used to investigate the molecular and cellular mechanisms in genetically tractable disease-relevant human cell types] [PMC free article] [PubMed] [Google Scholar]
44. Stefansson H, et al. CNVs conferring risk of autismor schizophrenia affect cognition in controls. Nature. 2014;505(7483):361–6. [PubMed] [Google Scholar]
Genetics of schizophrenia and relatives of patients with schizophrenia
The genetics of schizophrenia.
From the time of B. Morel's theory of degeneration and later, during the 19th and 20th centuries, psychiatrists from different countries have repeatedly expressed the idea that "dementia praecox" and schizophrenia should be considered a hereditary disease.
Frequent cases of schizophrenia in one family were explained by a genetic predisposition to this mental disorder. It has even been argued that hereditary burden of schizophrenia confirms its nosological integrity. nine0005
At the end of the 1930s, K. Luxenburger (1938) wrote: “Recent years have taught us, in any case, that the clinic and psychopathology have unsuccessfully tried to destroy the unity of schizophrenia. It must be considered, first of all, as a hereditary-biological unity. However, other psychiatrists, in particular H. Kallmann (1938), believed that it was necessary to single out the "marginal" predisposition with a lower and "nuclear" predisposition with a greater likelihood of developing schizophrenia. K. Luxenburger and H. Kallmann cited conflicting data regarding the concordance of schizophrenia in identical twins and spoke differently about the fatal role of the genotype in the genesis of schizophrenia. nine0005
Some psychiatrists have noted that in "states similar to schizophrenia" the prognosis is clearly more favorable than in "true schizophrenia", since in the first case there is only a "partial predisposition" in the form of weakness of the connective tissue or a tendency to develop tuberculosis. In this position, the attentive reader will notice the influence of E. Kraepelin, who wrote about the diagnostic significance of the outcome of schizophrenia.
According to a number of researchers of the first half of the 20th century, conditions resembling the clinical manifestations of schizophrenia require exogenous activation to a much greater extent than true schizophrenia. nine0005
It has been observed that “semi-burdened” or “semi-prone” to schizophrenia people are distinguished by oddities of character, unusual personality traits. Some of them, perhaps even significant, at different moments of life, with any illness or stress, reveal mild and, as a rule, short-term psychopathological symptoms (“sounding of symptoms”), which were also observed in the clinical picture of schizophrenia.
The proximity of a number of mental disorders in their clinical symptoms to the manifestations of schizophrenia led to the idea of the existence of its "atypical forms". K. Leonhard (1940) talked about the inheritance of "atypical schizophrenia" in a special way. At the same time, the thought expressed by him that “atypical forms of schizophrenia” should be distinguished by a greater hereditary burden seemed paradoxical.
In the middle of the 20th century, it became known that some variants of manic-depressive psychosis (“atypical psychoses”) and schizophrenia may have the same hereditary basis. These assumptions undermined the nosological independence of schizophrenia, but were most often refuted by the results of other studies. nine0005
Atypical endogenous psychoses, combining signs of both schizophrenia and manic-depressive psychosis, were described by domestic researchers in the third quarter of the 20th century under the name "periodic schizophrenia". At the same time, the results of genetic studies of "periodic schizophrenia" did not make it possible to recognize it as a separate nosological unit.
If in the first half of the 20th century most research on the genetics of schizophrenia was carried out from the position of hereditary homogeneity of the disease, then in the late 60s many psychiatrists criticized this approach (WHO, 1967).
In the middle of the 20th century, Japanese scientists, using a large amount of factual material, showed that "periodic schizophrenia" is characterized by a specific genotype that is not associated with a predisposition to other mental illnesses.
In the 1960s, some researchers believed that the predisposition or “deposit” for schizophrenia was transmitted in the genus by the type of autosomal recessive and intermediate inheritance, i. e. heterozygous carriers of this recessive "deposit", from the point of view of the phenotype, even "outwardly" often differ from persons who are completely free from the hereditary "deposit" (Galachyan A., 1962).
Due to the fact that the division of traits into dominant and recessive is quite artificial, it has been rightly suggested that many hereditary diseases, including schizophrenia, are characterized by both dominant and recessive types of inheritance.
The phenomena of incomplete dominance and incomplete recessivity are known, the same gene, dominant in a heterozygous individual in a homozygous state, has a quantitatively and qualitatively different effect. Examples of codominance indicate that the assignment of a phenotype to the category of recessive or dominant is largely determined by the sensitivity of the method for identifying the mechanisms of gene action. nine0005
It was assumed that the schizophrenic genotype manifests itself primarily as a disease of the brain, but, according to scientists of those years, it could also be detected in the disorder of the functions of other organs. Based on this hypothesis, J. Wyrsch., 1960, and a number of other authors concluded that hopes for quality care for patients with schizophrenia should be placed not on psychopathology, but on pathophysiology.
At one time, among psychiatrists, a case of schizophrenia in the famous four identical twins of girls, described in the monograph by D. Rosenthal et al., aroused great interest. (nineteen63). The girls' father was distinguished by his mental imbalance. All four girls studied normally at school, three of them finished it well, but at the age of 20-23, all the girls began to develop manifestations of schizophrenia, and very rapidly with signs of catatonia in the one who did not finish high school.
Many researchers assumed that in schizophrenia, the weakness of certain systems may be inherited and, in particular, the way they react physiologically to internal and external factors (Semenov S.F., 1962). Some scientists have argued that genetic disorders in various mental disorders may be identical.
The schizophrenia genetic spectrum typically included: latent schizophrenia, schizotypal disorder, schizoid personality disorder, and paranoid personality disorder.
In Russian psychiatry, V.P. Efroimson and M.E. Vartanian (1967).
Most researchers came to the conclusion that there is no reason to assume a genetic link between manic-depressive psychosis, "periodic schizophrenia", atypical endogenous psychoses and "true" process schizophrenia (Kunin A.Sh., 1970).
In the 1970s, the idea that different diseases are included in schizophrenia was confirmed by the facts of the discovery of genetic differences between paranoid schizophrenia and hebephrenia (Vinocur J., 1975).
For modern researchers in the genetics of schizophrenia, this area is of interest in three aspects: genetics can reveal the etiology of schizophrenia; the pharmacogenetic approach allows optimizing the therapeutic process, individually selecting drugs for its treatment and minimizing the side effects of drug therapy; the genetic research method allows you to get an answer to the question about the polymorphism of the clinical picture of schizophrenia (Sullivan P. Et al., 2006). nine0005
Main directions of genetic research in schizophrenia
- Studying the etiology of schizophrenia
- Study of the Genesis of Clinical Polymorphism of Schizophrenia
- Pharmacogenetic studies
Relatives of patients with schizophrenia
Modern genetics suggests that even general aspects of an adult's personality are genetically determined, for example, an increased level of anxiety, manifested, in particular, by anxiety about one's health and excessive excitement when it is necessary to make a decision in difficult situations. nine0005
Anxiety or calmness, shyness or impudence, the strength of instincts, the need and exactingness in their satisfaction, alertness, sensitivity to criticism, the degree of severity of disorganization of behavior in difficult circumstances, according to some geneticists, are also hereditarily determined. As proof of this point of view, information is given about identical twins who grew up in different conditions, but are similar to each other in terms of the above and other personal characteristics. nine0005
Recently, there is evidence that even the search for adventure and love of risk are partially associated with alleles in the locus of a certain gene (Viktor M., Ropper A., 2006).
Mental disorders in the genesis of which the factor of heredity plays a particularly important role
- Autism
- Hyperactivity
- Schizophrenia
- Mood disorders
At one time, employees of the pathophysiological laboratory of the Institute of Psychiatry of the USSR Academy of Medical Sciences demonstrated that a number of biochemical and immunological abnormalities found in patients with schizophrenia can also be detected in their relatives. It was, in particular, about such deviations as the ratio of lactate and pyruvate in the blood, the presence of altered forms of lymphocytes, distortion of immune responses, etc. (Vartanyan M.E., 1972).
At the same time, studies have shown that the range of personality anomalies among first-degree relatives is limited and usually limited to schizoid disorders (Shakhmatova-Pavlova I. V., 1975).
It was proposed to distinguish three main categories of relatives of patients with schizophrenia:
- persons with proper schizoid features, with a "vital tone", a background independent of the external environment, which determined the pace and intensity of mental activity; nine0056
- persons with schizoid features and a predominance of a pronounced emotional defect;
- schizoid personalities with distinct affective disorders (elevated mood background, bipolar phase change, seasonal depression).
Approximately 20-30% of first-degree relatives of patients with schizophrenia have so-called "spectral disorders", which are more or less attenuated symptoms of schizophrenia. These "weakened symptoms" most often appear in the form of sharpening of certain personality traits: isolation, increased vulnerability, "emotional dullness". nine0005
Japanese scientists in the study of cases of schizophrenia in childhood found a high incidence of schizoid psychopathy among the parents of children.
Variants of schizoid personalities among relatives of patients with schizophrenia
- Personalities with an altered "vital tone" ("a background of mental activity independent of the external environment")
- Individuals with signs of an "emotional defect" ("emotional dullness")
- Closed, sensitive personalities
According to I.V. Shakhmatova-Pavlova (1975) there is a schizophrenic continuum in the family plan, represented by a number of disorders (pronounced psychosis, erased forms, character anomalies, accentuated personality), and this continuum is in good agreement with the theory of the influence of a combination of factors on the pathogenesis of schizophrenia (Morkovkin V.M. , Kartelyshev A.V., 1988).
Some studies have found that relatives of patients with schizotypal traits diagnosed with schizotypal personality disorder score lower on certain cognitive tests than relatives without personality anomalies (Cannon. , 1994).
Features of the cognitive sphere of relatives of patients with schizophrenia
- Altered speed of psychomotor reactions
- Violation of short-term verbal and visual memory
- Attention volatility
- Features of abstract thinking (unusual formation of concepts, coding of information)
- Difficulties in building an action plan and consistent implementation of the set goals
- Difficulties of copying images
Modern studies of the cognitive sphere of relatives of patients with schizophrenia allow substantiating the position on the presence of independent cognitive syndromes in patients and in individuals with a high genetic risk of schizophrenia. These syndromes are associated with genes involved in the formation of various biochemical systems. It is assumed that the preservation of cognitive processes in some relatives of patients is explained by the successful compensation of primary disorders due to sufficient intellectual resources (Alfimova M. V., 2007). nine0005
C. Gilvarry et al. (2001) showed that in relatives of patients with schizophrenia, the severity of paranoid traits correlates with intelligence quotient, schizoid traits with the speed of psychomotor reactions, and schizotypal traits with verbal fluency.
>Patients with schizophrenia and their relatives often show similar features of cognitive processes.
An analysis of schizotypal traits leads to the idea that the disorganization of thinking and speech is associated with the stability of attention and the state of psychomotor functions, and the violation of interpersonal relationships is associated with the stability of attention and features of short-term verbal memory (Squires-Wheeler E., et al., 1997; Chen W., et al., 1998). At the same time, the relationship between schizotypal traits and cognitive impairment is detected in relatives of patients, but not in individuals without a hereditary burden of schizophrenia. As a result of the foregoing, it can be assumed that neurocognitive deficit reflects a hereditary predisposition to schizophrenia (Alfimova M. V., 2007). According to R. Asarnow et al. (2002). Moreover, neurocognitive deficit can be transmitted as an inherited trait in the families of patients, regardless of the presence of schizophrenia spectrum disorders. nine0005
Schizophrenia researchers have repeatedly tried to find signs that reflect the influence of the genotype predisposing to schizophrenia (“endophenotype”).
The term "endophenotype" in relation to schizophrenia was proposed by I. Gottesman and J. Schields (1972), who understood the "endophenotype" as an internal phenotype or trait that is intermediate between the clinical manifestations and the genotype of schizophrenia. In their later works, these authors identified a number of criteria according to which a trait could be considered an “endophenotype”: a trait is associated with a disease at the population level, it is an inherited trait, its severity practically does not depend on the state or severity of the disease, within families the endophenotype and disease cosegregate, in unaffected relatives of the patient, the endophenotype is found more often than in the general population. According to I. Gottesman and J. Schields (2003), other terms, such as "intermediate phenotype", "biological marker", "susceptibility marker", should be used to refer to those traits that do not necessarily reflect the genetic characteristics of the disease, and may be a manifestation of other factors affecting the onset and course of schizophrenia. nine0005
W. Kremen et al. (1994) concluded that people who are genetically predisposed to schizophrenia show the most pronounced deviations in the cognitive sphere. First of all, we are talking about the stability of attention, perceptual-motor speed, concept formation, features of abstract thinking, context processing, control of mental processes and coding. In addition, a number of researchers have identified disorders in visual and verbal associative memory in relatives of patients with schizophrenia (Trubnikov V.I., 1994).
M. Appels (2002) found cognitive changes in parents of patients with schizophrenia similar to changes in the patients themselves, but expressed to a lesser extent.
M. Sitskoorn et al. (2004), based on the results of a meta-analysis, showed that for integral indicators of the reproduction of verbal information and executive functions, the effect size (the degree of difference between the mean value of a trait in a group of relatives of patients with schizophrenia and normative indicators - d) is quite pronounced (d = 0.51), and for indicators of attention it is uninformative (d = 0.28). nine0005
Among the memory subprocesses, the maximum differences between relatives of patients and the control group were found when analyzing the results of direct reproduction of a list of words (d = 0.65), immediate and delayed reproduction of text (d = 0.53 and 0.52), the minimum - for delayed reproduction of visual information (0.32) (Whyte M. Et al., 2005).
The results of another meta-analysis showed that in relatives of patients with schizophrenia, the most informative test was semantic verbal fluency, as well as tests for copying figures from a model and memorizing a list of words. The researchers noted that the severity of these disorders in relatives of patients with schizophrenia is influenced by age and education and is not influenced by personality type and degree of relationship (Snitz B. et al., 2006). These studies also looked at the prevalence of cognitive impairment. It turned out that relatives of patients with schizophrenia in approximately 70% of cases show mild cognitive impairment, reminiscent of disorders of the cognitive sphere of patients with schizophrenia. The groups of relatives and controls overlapped by 70%, while the groups of patients and controls overlapped by only 45%. nine0005
At the same time, it should be noted that cognitive impairments that occur in relatives of patients with schizophrenia are only relatively specific to this mental disorder. They are also registered among relatives of patients with affective disorders, which to some extent may indicate the presence of a common, albeit weakly expressed, genetic predisposition to these mental disorders.
Most clearly, cognitive disorders in a significant part of the relatives of patients manifest themselves in violation of executive functions that require deep semantic processing of information, and the reproduction of verbal information associated with a large load on memory. These disorders have varying degrees of specificity, genetic determination and are associated in different ways with schizotypal personality traits (Alfimova M.V., 2007). nine0005
It is interesting to note that studies of a number of mathematically gifted children revealed features of the cognitive process found in patients with schizophrenia. Indeed, according to the statements of many teachers, children who are prone to mathematics are distinguished by strange behavior, originality of views and isolation.
According to our data, in relatives of patients with paranoid schizophrenia, there is a tendency to delusion, there is a special viscosity of thinking, a tendency to excessive detail, a desire to attribute the actions of people around them to one's own account. It is no coincidence that in delirium, unlike hallucinations, it is not possible to find sufficiently distinct changes in certain brain structures. Probably, in schizophrenia, under the influence of a pathological process, an already existing tendency to delusional formation (a tendency to easily generate overvalued ideas) develops into a real delirium. nine0005
Symptoms of the prodromal period of schizophrenia can be quite difficult to distinguish from the personality traits of relatives of patients with schizophrenia. It can be assumed that if there is a genetic predisposition to schizophrenia, which usually manifests itself at the phenotype level, then under the influence of a number of factors (changes in the activity of the endocrine glands, prolonged psycho-traumatic experiences, autoimmune processes, etc.), the disease can clearly manifest itself. At the same time, it can be assumed that the prodromal period of schizophrenia will not necessarily end with the manifestation of diseases, and in this case, mild personality changes, mild neurophysiological and psychophysiological abnormalities will remain only in the form of a "trace" of the outbreak of the pathological process. This is partly noticeable in the example of the so-called "acquired schizoidization" of the individual. nine0005
Morphological changes in the brain of patients with schizophrenia, in particular the expansion of the lateral ventricles, in some cases are similar to structural changes in the brain of patients' relatives. Danish scientists have shown that healthy relatives of patients with schizophrenia often have not only dilated lateral ventricles, but also an increase in the third ventricle of the brain, a decrease in the size of the thalamus, a decrease in the volume of the frontal and parietal lobes. As a result of the foregoing, anatomical changes in certain brain structures can be considered as a genetic risk factor. nine0005
Structural and functional changes in the brain, most often recorded in relatives of patients with schizophrenia
- Expansion of the lateral and third ventricles of the brain
- Thalamus reduction
- Decrease in the volume of the frontal and parietal lobes
- Eye muscle dysmotility (antisaccade test)
- Changes in the bioelectrical activity of the basal areas of the frontal and left temporal lobes
- Deficiency in prepulse inhibition reflecting a deficiency in the GABA system
As is known, the test for atysaccades (eye movement following a tracking object from the periphery of the visual field to the center), concerning the dysmotility of the eye muscles, is considered a fairly specific phenomenon for schizophrenia, reflecting a hereditary predisposition to this disease. Mild anomalies in tracking eye movements and mild deviations from the norm of neurophysiological and psychophysiological parameters are often observed in relatives of patients with schizophrenia.
It is interesting to note that foreign researchers, using electrodes implanted for several months, determined that in the basal areas of the frontal lobes of the brain in patients and their relatives, equally deviant curves are observed. nine0005
EEG studies showed a high probability of inheritance of the main rhythms, especially in the slow-wave part of the spectrum with a relative maximum in the occipital, left middle-temporal, and right central leads.
An electrophysiological study of the bioelectrical activity of the brain revealed patterns characteristic of schizophrenia with a high heritability of the main rhythms alpha, beta-1, beta-2, mainly in the leads of the left hemisphere (heritability 42-85%), and slow rhythms, mainly theta waves, in left parietal, central and upper parts of the frontal leads (52-72%) (Kudlaev M. V., Kudlaev S.V.). nine0005
According to V.P. Efroimson and L.G. Kalmykova (1970), the risk of schizophrenia for the general population is approximately 0.85%, for the patient's siblings - 10%, for half-sibs - 3.5%, for children - 14%, parents - 6%. At the same time, in a marriage between two patients with schizophrenia, the risk of morbidity for children varies in a wide range from 38 to 68%, and the risk for the patient's siblings increases sharply if one, and even more so two parents suffer from schizophrenia
According to N.S. Natalevich (1970), if a mother suffers from schizophrenia, then the probability of developing this disease in her child is 13.3%, if the father, then only 5%.
Studies by L. Gottesman (2000) (Table 5) showed that the risk of developing schizophrenia increases from about 1% in the general population to 50% in the offspring of two parents of schizophrenic patients (a similar figure for identical schizophrenic twins).
According to L. Erlimeyer-Kimling (1968), in a family with one of the parents with schizophrenia, the probability of children getting sick is 12-16%, and in the case of diseases of both parents, no more than 30-46%. nine0005
According to V.A. Milev and V.D. Moskalenko (1988), the frequency of schizophrenia for complete siblings of probands approaches 16%, while for half-sibs it approaches 6%. Researchers provide evidence that children of mothers with schizophrenia almost always show certain disorders of social adaptation and develop schizophrenia in more than 40% of cases (Heston L., 1966) or 5 times more often than children of fathers with schizophrenia (Ozerova N .I. et al., 1983).
Table 6. Risk of schizophrenia for relatives of patients
| Relationship | Risk (%) |
| Population as a whole | 0.5-1% |
| Spouses | 1-2% |
| Relatives of the 3rd degree of kinship | 1-2% |
| Siblings of proband's parents | 1-2% |
| Nephews | 3-4% |
| Grandchildren | 4-5% |
| Parents | 5-6% |
| Sibs | 8-9% |
| Children of one sick parent | 12-13% nine0220 |
| Siblings with one of the sick parents | 15-17% |
| Dizygotic twins | 13-17% |
| Monozygotic twins | 47-48% |
| Children with two sick parents | 45-46% |
Of interest is the comparative percentage of concordance of mental disorders in pairs of twins. For monozygotic twins with obsessive-compulsive disorder, it reaches 87%, with bipolar affective disorder - 79%, with schizophrenia and alcoholism - 59%. For heterozygous twins with obsessive-compulsive disorder, it is 47%, with bipolar affective disorder - 19%, with schizophrenia - 15%, with alcoholism - 36% (Table 7) (Muller N., 2001).
Table 7. Concordance of mental disorders in twins (adapted from Muller N., 2001) Mental disorders Monozygotic Heterozygous twins (%) Obsessive-compulsive disorder 87% 47% Bipolar affective disorder 79% 19% Schizophrenia 59% 15% Alcoholism 50% 36% Many researchers have emphasized that heredity is reflected in the type of course of schizophrenia. In the "parents-children" group, the prevalence of a continuous course was established. Usually, the differences were manifested in the earlier onset of the disease and its aggravation as it progressed. In 80% of cases, the similarity of clinical attacks of diseases was revealed. nine0005 Many authors drew attention to the high concordance of premorbid personality traits and a certain relationship between these features and the course of schizophrenia, which was equally noted in identical and fraternal twins. The similarity of reactions to medicinal substances and the results of therapy (pharmacogenetics) Lifshits E.Ya., 1970) was also revealed. Return to Table of Contents Schizophrenia is one of the most mysterious and complex diseases, and in many ways. It is difficult to diagnose - there is still no consensus on whether this disease is one or many similar to each other. It is difficult to treat it - now there are only drugs that suppress the so-called. The importance of this work is not even that almost every hundredth person on the planet suffers from schizophrenia, and progress in this area should at least radically simplify diagnosis, even if a good medicine cannot be created right away. First some raw facts. Schizophrenia is severe, chronic, leading to disability mental illness, usually affecting people at a young age age. From her about 50 million people suffer around the world (slightly less than 1% of the population). The disease is accompanied by apathy, lack of will, often hallucinations, delusions, disorganization of thinking and speech, motor disorders. Symptoms are usually cause social isolation and decrease in performance. elevated risk of suicide in patients with schizophrenia, and also concomitant somatic diseases lead to the fact that the general their life expectancy is decreasing for 10-15 years. Latest half a century was a time of rapid progress in many areas of medicine, however this progress has hardly affected prevention and treatment of schizophrenia. Not Lastly, this is due to that we still do not have a clear ideas about the violation of what biological processes is cause of the development of the disease. Such lack of understanding has led to time of appearance on the market of the first the antipsychotic drug chlorpromazine (trade name: "Aminazine") more 60 years ago there was still no quality changes in the treatment of the disease. Everything is now existing approved for treatment schizophrenia antipsychotics (both typical, including chlorpromazine and atypical) have the same basic mechanism actions: they reduce the activity of dopamine receptors that eliminates hallucinations and bullshit, but unfortunately weak affects on negative symptoms like apathy, lack of will, thinking disorders etc. nine0215
Insanity Inherited
positive symptoms (like delirium), but they do not help return the person to a full life. Schizophrenia is difficult to study - no other animal except humans suffers from it, so there are almost no models for studying it. Schizophrenia is very difficult to understand from a genetic and evolutionary point of view - it is full of contradictions that biologists cannot yet resolve. However, the good news is that in recent years things have finally seemed to get off the ground. We have already written in detail about the history of the discovery of schizophrenia and the first results of its study by neurophysiological methods. This time we will talk about how scientists are looking for the genetic causes of the disease. nine0005
The importance of genetic research lies in the fact that they are already changing our understanding of the fundamental mechanisms of inheritance of complex traits. If scientists still manage to understand how such a complex disease as schizophrenia can “hide” in our DNA, this will mean a radical breakthrough in understanding the organization of the genome. And the significance of such work will go far beyond clinical psychiatry. nine0005
In addition, patients with schizophrenia have fewer children men have an average of 75 percent, women — by 50 percent. nine0005
About the side effects, we even we don't mention. General disappointment in schizophrenia research is manifested in the fact that pharmaceutical companies have long been reducing funding for the development of antipsychotics - and this despite the fact that the total number of clinical trials is only growing. However hope for clarification of the causes schizophrenia came with a rather unexpected sides - she associated with unprecedented progress in molecular genetics. nine0005
Collective responsibility
More pioneers of schizophrenia noticed that the risk of getting sick closely tied with the presence of sick relatives. Attempts to establish mechanism inheritance schizophrenia were taken almost immediately after the rediscovery of Mendel's laws, at the very beginning of the 20th century. However, unlike from many other diseases, schizophrenia no way did not want fit into simple Mendelian models. Despite for high heritability, link her With one or more genes it turned out so by the middle of the century all become more popular so-called. psychogenic theories of disease development. AT agree with extremely popular by the middle of the century psychoanalysis, these theories explained the apparent heritability of schizophrenia is not genetics, but the features of education and unhealthy atmosphere inside families. Appeared even such a thing as "schizophrenogenic parents". nine0005
However this theory, despite its popularity, lived for a short time. final point on the question of whether schizophrenia is a hereditary disease, carried out psychogenetic studies, carried out already in 60-70s. These were primarily twin studies, and research on foster children. essence twin studies is compared the likelihood of some sign - in this case, development diseases - in identical and fraternal twins. Because the difference in the action of the environment on the twins does not depend from whether they are identical or heterozygous, then differences in these probabilities must occur mainly from the fact that they are identical twins are genetically identical, and fraternal have, on average, only half of the options genes. nine0005
B case of schizophrenia, it turned out that concordance of identical twins more than 3 times higher than concordance fraternal: for first it is approximately 50 percent, a for second - less 15 percent. These words should be understood as follows: if you have a sufferer schizophrenic monozygotic twin brother then you themselves get sick with a 50 percent chance. If you and your brother are fraternal twins, then the risk of getting sick is not over 15 percent. Theoretical calculations that additionally take into account the prevalence schizophrenia in the population, give an estimate contribution of heritability in the development of the disease level of 70-80 percent. For comparisons, approximately the same is inherited height and body mass index are signs, which have always been considered closely related with genetics. By the way, as it turned out later, just as high heritability is characteristic of three of the other four major mental illnesses: attention deficit disorder and hyperactivity, bipolar disorder and autism. nine0005
Results twin studies completely confirmed in the study of children, which born to patients with schizophrenia and were adopted at an early age infancy healthy adoptive parents. It turned out that the risk of getting schizophrenia they have not reduced compared to children, raised by their schizophrenic parents, what definitely indicates on the key role of genes in etiology.
I here we come to one of the most mysterious features of schizophrenia. The fact is that if she is so strong inherited and at the same time very negatively affects the wearer's fitness (Recall that patients with schizophrenia leave at least half as much offspring than healthy people), then how should she manages to persist in the population for at least multiple hundreds of thousands of years? it contradiction around which in in many ways the main struggle is going on between different theories called "evolutionary paradox of schizophrenia»
Until recently, it was completely unclear to scientists which particular features of the genome of schizophrenic patients predetermine the development of the disease. For decades, there has been a heated debate not even about which genes are changed in patients with schizophrenia, but about what is the general genetic "architecture" of the disease. nine0005
This means the following. The genomes of individual people are very similar to each other, with differences averaging less than 0.1 percent of nucleotides. Some of these distinguishing features of the genome are quite widespread in the population. It is conventionally considered that if they occur in more than one percent of people, they can be called common variants or polymorphisms. These common variants are believed to have appeared in the human genome over 100,000 years ago, before the first migration from Africa of the ancestors of modern humans, so they are commonly found in most human subpopulations. Naturally, in order to exist in a significant part of the population for thousands of generations, most of the polymorphisms should not be too harmful to their carriers. nine0005
However, in the genome of each of the people there are other genetic features - younger and rarer. Most of them do not provide carriers with any advantage, so their frequency in the population, even if they are fixed, remains insignificant. Many of these traits (or mutations) have a more or less pronounced negative effect on fitness, so they are gradually removed by negative selection. Instead, as a result of a continuous mutation process, other new harmful variants appear. In sum, the frequency of any of the new mutations almost never exceeds 0.1 percent, and such variants are called rare. nine0005
So, the architecture of a disease means which genetic variants - common or rare, having a strong phenotypic effect, or only slightly increasing the risk of developing a disease - predetermine its occurrence. It is around this issue that, until recently, the main debate about the genetics of schizophrenia was conducted.
The only fact indisputably established by molecular genetic methods regarding the genetics of schizophrenia over the last third of the 20th century is its incredible complexity. Today it is obvious that the predisposition to the disease is determined by changes in dozens of genes. At the same time, all the "genetic architectures" of schizophrenia proposed during this time can be combined into two groups: the "common disease - common variants" (CV) model and the "common disease - rare variants" model (common disease - rare variants, RV). Each of the models gave its own explanation of the "evolutionary paradox of schizophrenia." nine0005
RV vs. CV
According to the CV model, the genetic substrate of schizophrenia is a set of genetic traits, a polygene, akin to that which determines the inheritance of quantitative traits such as height or body weight. Such a polygene is a set of polymorphisms, each of which only slightly affects the physiology (they are called "causal", because, although not alone, they lead to the development of the disease). To maintain a rather high incidence rate characteristic of schizophrenia, it is necessary that this polygene consists of common variants - after all, it is very difficult to collect many rare variants in one genome. Accordingly, each person has dozens of such risky variants in his genome. In sum, all causal variants determine the genetic predisposition (liability) of each individual to the disease. It is assumed that for qualitative complex features, such as schizophrenia, there is a certain threshold value of predisposition, and only those people whose predisposition exceeds this threshold value develop the disease. nine0005
For the first time, such a polygenic model of schizophrenia was proposed in 1967 by one of the founders of modern psychiatric genetics, Irving Gottesman, who also made a significant contribution to proving the hereditary nature of the disease. From the point of view of adherents of the CV model, the persistence of a high frequency of causal variants of schizophrenia in the population over many generations can have several explanations. First, each individual such variant has a rather minor effect on the phenotype, such "quasi-neutral" variants may be invisible to selection and remain common in populations. This is especially true for populations with low effective size, where the influence of chance is no less important than the pressure of selection - this includes the population of our species. nine0005
On the other hand, there have been suggestions about the presence in the case of schizophrenia of the so-called. balancing selection, i.e., the positive effect of "schizophrenic polymorphisms" on healthy carriers. It's not that hard to imagine. It is known, for example, that schizoid individuals with a high genetic predisposition to schizophrenia (of which there are many among close relatives of patients) are characterized by an increased level of creative abilities, which can slightly increase their adaptation (this has already been shown in several works). Population genetics allows for a situation where the positive effect of causal variants in healthy carriers may outweigh the negative consequences for those people who have too many of these "good mutations", which led to the development of the disease. nine0005
The second basic model of the genetic architecture of schizophrenia is the RV model. She suggests that schizophrenia is a collective concept and that each individual case or family history of the disease is a separate quasi-Mendelian disease associated in each individual case with unique changes in the genome. In this model, causal genetic variants are under very strong selection pressure and are quickly removed from the population. But since a small number of new mutations occur in each generation, a certain balance is established between selection and the emergence of causal variants. nine0005
On the one hand, the RV model can explain why schizophrenia is very well inherited, but its universal genes have not yet been found: after all, each family inherits its own causal mutations, and there are simply no universal ones. On the other hand, if we are guided by this model, then we have to admit that mutations in hundreds of different genes can lead to the same phenotype. After all, schizophrenia is a common disease, and the occurrence of new mutations is rare. For example, data on sequencing of father-mother-child triplets show that in each generation, only 70 new single-nucleotide substitutions occur per 6 billion nucleotides of the diploid genome, of which, on average, only a few can theoretically have any effect on the phenotype, and mutations of other types is an even rarer occurrence. nine0005
However, some empirical evidence indirectly supports this model of the genetic architecture of schizophrenia. For example, in the early 1990s, it was discovered that about one percent of all patients with schizophrenia had a microdeletion in one of the regions of the 22nd chromosome. In the vast majority of cases, this mutation is not inherited from parents, but occurs de novo during gametogenesis. One in 2,000 people is born with this microdeletion, which leads to a variety of abnormalities in the body, called "DiGeorge syndrome." Those suffering from this syndrome are characterized by severe impairment of cognitive functions and immunity, they are often accompanied by hypocalcemia, as well as problems with the heart and kidneys. A quarter of people with DiGeorge syndrome develop schizophrenia. It would be tempting to suggest that other cases of schizophrenia are due to similar genetic disorders with catastrophic consequences. nine0005
Another empirical observation indirectly confirming the role of de novo mutations in the etiology of schizophrenia is the relationship of the risk of getting sick with the age of the father. So, according to some data, among those whose fathers were over 50 years old at the time of birth, there are 3 times more patients with schizophrenia than among those whose fathers were under 30. de novo mutations. Such a connection, for example, has long been established for sporadic cases of another (monogenic) hereditary disease - achondroplasia. This correlation has most recently been confirmed by the above triplet sequencing data: number de novo mutations are associated with paternal age but not with maternal age. According to the calculations of scientists, a child on average receives 15 mutations from the mother, regardless of her age, and from the father - 25 if he is 20 years old, 55 if he is 35 years old and over 85 if he is over 50. That is, the number of de novo mutations in the child's genome increases by two with each year of the father's life.
Together, these data seemed to indicate quite clearly the key role of de novo mutations in the etiology of schizophrenia. However, the situation actually turned out to be much more complicated. Even after the separation of the two main theories, for decades the genetics of schizophrenia stagnated. Almost no reliable reproducible evidence has been obtained in favor of one of them. Neither about the general genetic architecture of the disease, nor about specific variants that affect the risk of developing the disease. A sharp jump has occurred over the past 7 years and it is associated primarily with technological breakthroughs. nine0005
In search of genes
The sequencing of the first human genome, the subsequent improvement of sequencing technologies, and then the advent and widespread introduction of high-throughput sequencing finally made it possible to gain a more or less complete understanding of the structure of genetic variability in the human population. This new information immediately began to be used for a full-scale search for genetic determinants of predisposition to certain diseases, including schizophrenia. nine0005
Similar studies are built like this. First, a sample of unrelated sick people (cases) and a sample of unrelated healthy individuals (controls) of approximately the same size are collected. All these people are determined by the presence of certain genetic variants - just in the last 10 years, researchers have the opportunity to determine them at the level of entire genomes. Then, the frequency of occurrence of each of the identified variants is compared between groups of sick people and a control group. If at the same time it is possible to find a statistically significant enrichment of one or another variant in carriers, it is called an association. Thus, among the vast number of existing genetic variants are those that are associated with the development of the disease. nine0005
An important measure characterizing the effect of a disease-associated variant is the OD (odds ratio, risk ratio), which is defined as the ratio of the chances of getting sick in the carriers of this variant compared to those people who do not have it. If the OD value of a variant is 10, this means the following. If we take a random group of carriers of the variant and an equal group of people who do not have this variant, it turns out that in the first group there will be 10 times more patients than in the second. At the same time, the closer the OD is to one for a given variant, the larger the sample is needed in order to reliably confirm that the association really exists - that this genetic variant really affects the development of the disease. nine0005
Such work has now made it possible to detect more than a dozen submicroscopic deletions and duplications associated with schizophrenia throughout the genome (they are called CNV - copy number variations, one of the CNVs just causes the DiGeorge syndrome already known to us). For the CNVs that have been found to cause schizophrenia, the OD ranges from 4 to 60. These are high values, but due to their extreme rarity, even in total, they all explain only a very small part of the heritability of schizophrenia in the population. What is responsible for the development of the disease in everyone else? nine0005
After relatively unsuccessful attempts to find CNVs that would cause disease not in a few rare cases, but in a significant part of the population, supporters of the "mutation" model had high hopes for another type of experiment. They compare in patients with schizophrenia and healthy controls not the presence of massive genetic rearrangements, but the complete sequences of genomes or exomes (the totality of all protein-coding sequences). Such data, obtained using high-throughput sequencing, makes it possible to find rare and unique genetic features that cannot be detected by other methods. nine0005
Cheaper sequencing has made it possible in recent years to conduct experiments of this type on rather large samples, including several thousand patients and the same number of healthy controls in recent studies. What is the result? Alas, so far only one gene has been found, in which rare mutations are reliably associated with schizophrenia - this is the SETD1A gene, which encodes one of the important proteins involved in the regulation of transcription. As with CNV, the problem is the same: mutations in gene SETD1A cannot explain much of the heritability of schizophrenia due to the fact that they are simply very rare.
There are indications that there are other rare and unique variants that influence susceptibility to schizophrenia. And further increase in samples in experiments using sequencing should help to find some of them. However, while the study of rare variants may still provide some valuable information (especially this information will be important for creating cellular and animal models of schizophrenia), most scientists now agree that rare variants play only a minor role in heritability. schizophrenia, and the CV model is much better at describing the genetic architecture of the disease. The confidence in the correctness of the CV model came first of all with the development of GWAS-type studies, which we will discuss in detail in the second part. In short, studies of this type have uncovered the very common genetic variability that describes a large proportion of the heritability of schizophrenia, the existence of which was predicted by the CV model. nine0005
Additional support for the CV model for schizophrenia is the relationship between the level of genetic predisposition to schizophrenia and so-called schizophrenia spectrum disorders. Even early researchers of schizophrenia noticed that among the relatives of patients with schizophrenia, there are often not only other patients with schizophrenia, but also "eccentric" personalities with oddities of character and symptoms similar to schizophrenic, but less pronounced. Subsequently, such observations led to the concept that there is a whole set of diseases that are characterized by more or less pronounced disturbances in the perception of reality. This group of diseases is called the schizophrenia spectrum disorder. In addition to various forms of schizophrenia, these include delusional disorders, schizotypal, paranoid and schizoid personality disorders, schizoaffective disorder and some other pathologies. Gottesman, proposing his polygenic model of schizophrenia, suggested that people with subthreshold values of predisposition to the disease may develop other pathologies of the schizophrenic spectrum, and the severity of the disease correlates with the level of predisposition. nine0005
If this hypothesis is correct, it is logical to assume that the genetic variants found to be associated with schizophrenia would also be enriched among people suffering from schizophrenia spectrum disorders. To assess the genetic predisposition of each individual, a special value is used, called the level of polygenic risk (polygenic risk score). The level of polygenic risk takes into account the total contribution of all common risk variants identified in the GWAS, present in the genome of a given person, to the predisposition to the disease. It turned out that, as predicted by the CV model, the values of the polygenic risk level correlate not only with schizophrenia itself (which is trivial), but also with other diseases of the schizophrenia spectrum, and higher levels of polygenic risk correspond to severe types of disorders. nine0005
And yet one problem remains - the phenomenon of "old fathers". If much of the empirical evidence supports the polygenic model of schizophrenia, how does one reconcile with it the long-established association between age at fatherhood and children's risk of developing schizophrenia?
An elegant explanation of this phenomenon in terms of the CV model was once put forward. It has been suggested that late fatherhood and schizophrenia are not cause and effect, respectively, but are two consequences of a common cause, namely the genetic predisposition of late fathers to schizophrenia. On the one hand, a high level of susceptibility to schizophrenia may correlate in healthy men with later fatherhood. On the other hand, it is clear that a father's high predisposition predetermines an increased likelihood that his children will develop schizophrenia. It turns out that we can deal with two independent correlations, which means that the accumulation of mutations in the precursors of spermatozoa in men can have almost no effect on the development of schizophrenia in their descendants. Recent modeling results that take into account epidemiological data, as well as recent molecular data on the frequency of de novo mutations, are in good agreement with precisely this explanation of the “old fathers” phenomenon.
Thus, at the moment we can assume that there are almost no convincing arguments in favor of the “mutational” RV model of schizophrenia.