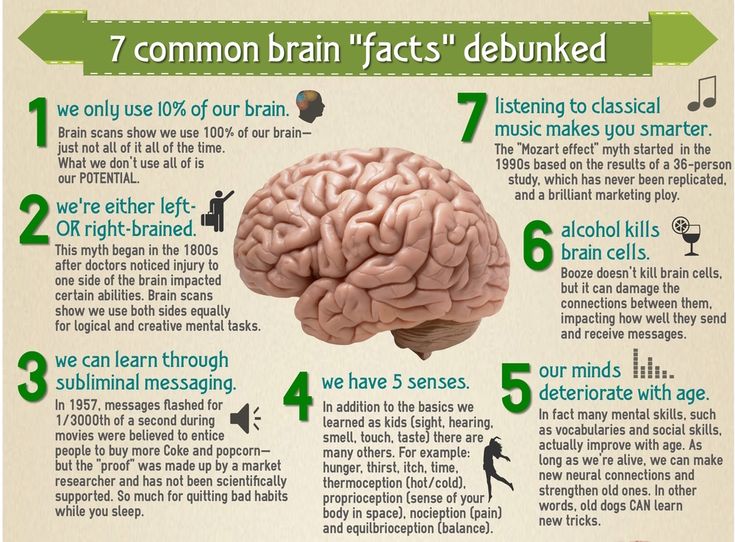
Does valacyclovir cause constipation
Common and Rare Side Effects for Valtrex
COMMON side effects
If experienced, these tend to have a Severe expression i
Sorry, we have no data available. Please contact your doctor or pharmacist.
If experienced, these tend to have a Less Severe expression i
INFREQUENT side effects
If experienced, these tend to have a Severe expression i
If experienced, these tend to have a Less Severe expression i
RARE side effects
If experienced, these tend to have a Severe expression i
If experienced, these tend to have a Less Severe expression i
Full Drug Information
Free RX Coupon
Save up to 80% on your prescriptions.
Available coupons
Save up to 80% on your prescription with WebMDRx
Related Links
Drug Survey
Are you currently using Valtrex?
This survey is being conducted by the WebMD marketing sciences department.
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.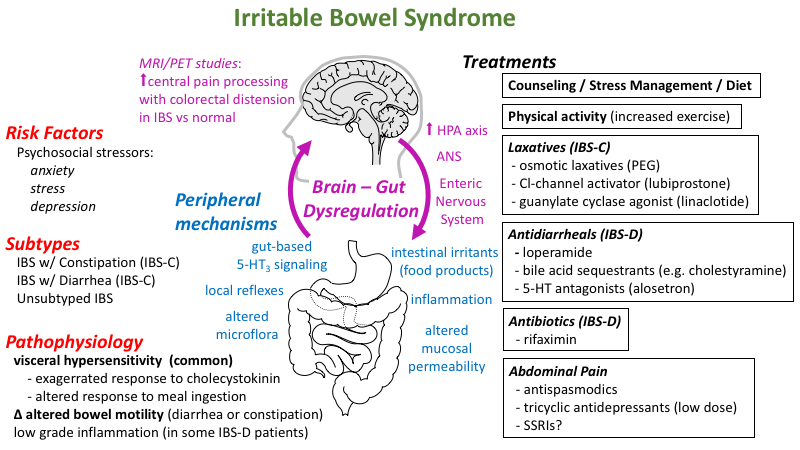
Side Effects, Dosage, Uses, and More
Highlights for valacyclovir
- Valacyclovir oral tablet is available as a brand-name drug and a generic drug. Brand name: Valtrex.
- Valacyclovir comes only as a tablet you take by mouth.
- Valacyclovir oral tablet is used to treat viral infections caused by a group of viruses called herpes simplex viruses. It’s used to treat cold sores (oral herpes), shingles, or chickenpox. It’s also used to treat or prevent flare-ups of genital herpes.
- Blood disorders warning: For certain people, this drug can cause thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS). These conditions cause a severely low level of red blood cells and platelets in your body. TTP or HUS can result in death. You’re at risk of these problems if you’ve had a bone marrow or a kidney transplant. You’re also at risk if you have advanced HIV or AIDS.
- Kidney failure warning: In some cases, this drug can cause your kidneys to stop working.
 This can occur if you’re on a high dose of this medication and have existing kidney problems. It can also occur if you’re taking other drugs that can harm your kidneys, if you’re not well hydrated, or if you’re over the age of 65 years.
This can occur if you’re on a high dose of this medication and have existing kidney problems. It can also occur if you’re taking other drugs that can harm your kidneys, if you’re not well hydrated, or if you’re over the age of 65 years. - Effects on the central nervous system warning: If you have kidney disease or use this drug at higher doses than your doctor prescribes, it can build up in your body. High levels of this drug can cause serious side effects that impact your brain. Symptoms can include hallucinations (seeing or hearing things that aren’t real) or delusions (believing things that aren’t true). They can also include agitation, confusion, or seizures. If you have any of these side effects, stop taking this drug. Call 911 right away or go to the nearest emergency room.
Valacyclovir is a prescription drug. It comes in the form of a tablet you take by mouth.
Valacyclovir is available as a brand-name drug called Valtrex. It’s also available as a generic drug. Generic drugs usually cost less than the brand-name version. In some cases, they may not be available in every strength or form as the brand-name drug.
Generic drugs usually cost less than the brand-name version. In some cases, they may not be available in every strength or form as the brand-name drug.
This drug may be used as part of a combination therapy. This means you may need to take it with other medications.
Why it’s used
Valacyclovir is used to treat viral infections caused by a group of viruses called herpes simplex viruses. These infections include oral and genital herpes, shingles, and chickenpox.
- Oral herpes causes cold sores. These are small, painful sores that you can get in or around your mouth. Cold sores can be spread by kissing or other physical contact with the infected area of the skin.
- Genital herpes is a sexually transmitted disease. This means it’s spread through sexual contact. Symptoms include small, painful blisters on the genital area. You can spread genital herpes to your sexual partner even when you don’t have any symptoms. This drug is used to treat or prevent flare-ups of genital herpes in people with normal immune systems, or in people with HIV.

- Shinglesis caused by the same virus that causes chickenpox (varicella zoster). Symptoms of shingles include small, painful blisters that appear on the skin. Shingles can occur in people who have already had chickenpox. It can also spread to people who have not had chickenpox before through contact with the infected skin.
- Chickenpoxcauses an itchy rash of small, red bumps that can look like pimples or insect bites. The rash can spread almost anywhere on the body. Chickenpox can also cause flu-like symptoms, such as fever or tiredness. This drug is used to treat chickenpox in children ages 2 to18 years who have a normal immune system.
How it works
Valacyclovir belongs to a class of drugs called antiviral drugs. A class of drugs is a group of medications that work in a similar way. These drugs are often used to treat similar conditions.
The herpes virus spreads in your body by creating more of its cells.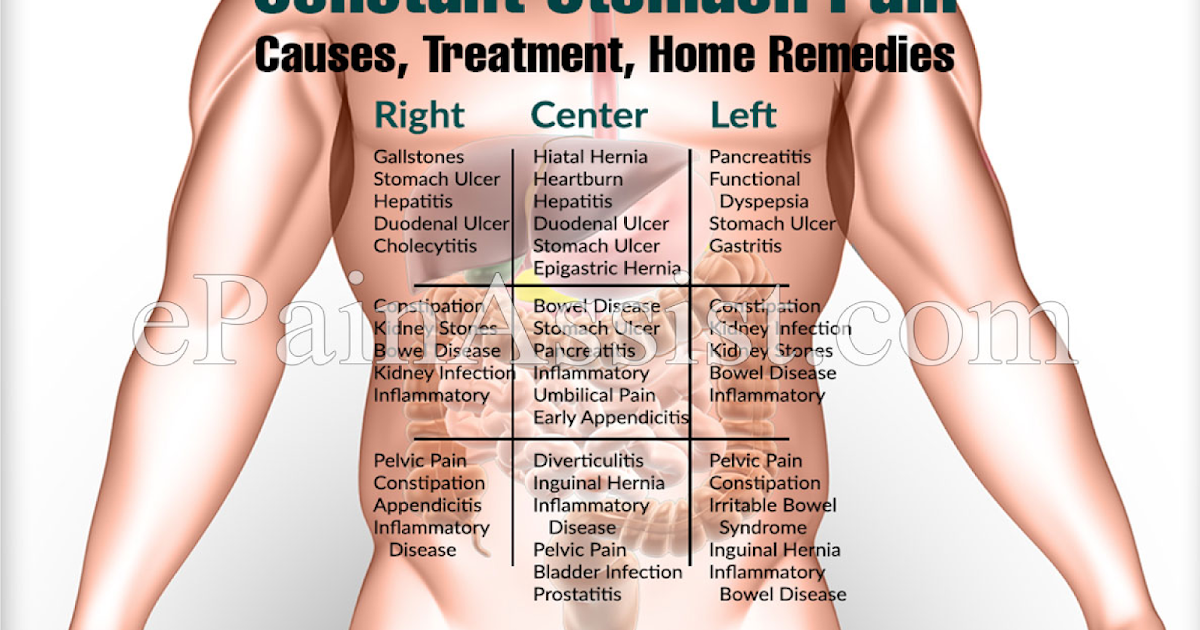 Valacyclovir works by making it harder for the herpes virus to multiply (make more cells) in your body.
Valacyclovir works by making it harder for the herpes virus to multiply (make more cells) in your body.
This drug does not cure herpes infections. The herpes virus may still live in your body after treatment. This means the infection may occur again at a later time even after the symptoms of the first infection are gone. However, this drug can help prevent such a re-infection at a later time.
Valacyclovir oral tablet doesn’t cause drowsiness, but it can cause other side effects.
More common side effects
The more common side effects of valacyclovir can include:
- headache
- nausea
- vomiting
- dizziness
- pain in your stomach area
If these effects are mild, they may go away within a few days or a couple of weeks. If they’re more severe or don’t go away, talk to your doctor or pharmacist.
Serious side effects
Call your doctor right away if you have serious side effects. Call 911 if your symptoms feel life-threatening or if you think you’re having a medical emergency. Serious side effects and their symptoms can include the following:
Serious side effects and their symptoms can include the following:
- Kidney failure. Symptoms can include:
- severe drowsiness
- urinating less than usual
- swelling in your legs, ankles, or feet
- Unusual mood or behavior. Symptoms can include:
- aggressive behavior
- unsteady or shaky movements
- confusion
- hallucinations
- seizures
- coma
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs affect each person differently, we cannot guarantee that this information includes all possible side effects. This information is not a substitute for medical advice. Always discuss possible side effects with a healthcare provider who knows your medical history.
Reducing your risk of spreading herpesUsing this medication daily may help lower the risk of spreading this disease to your sexual partner.
However, you should not have sexual contact with your partner when you have any symptoms of an outbreak of genital herpes. Even if you use safer sex practices such as using a condom, you can still spread genital herpes. Talk with your doctor for more information about how to have safer sex.
An interaction is when a substance changes the way a drug works. This can be harmful or prevent the drug from working well. To help prevent interactions, your doctor should manage all of your medications carefully. Be sure to tell your doctor about all medications, vitamins, or herbs you’re taking.
To find out how valacyclovir oral tablet might interact with something else you’re taking, talk to your doctor or pharmacist.
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs interact differently in each person, we cannot guarantee that this information includes all possible interactions. This information is not a substitute for medical advice. Always speak with your healthcare provider about possible interactions with all prescription drugs, vitamins, herbs and supplements, and over-the-counter drugs that you’re taking.
Always speak with your healthcare provider about possible interactions with all prescription drugs, vitamins, herbs and supplements, and over-the-counter drugs that you’re taking.
This drug comes with several warnings.
Allergy warning
This drug can cause a severe allergic reaction. Symptoms can include:
- trouble breathing
- swelling of your throat or tongue
If you develop these symptoms, call 911 or go to the nearest emergency room.
Don’t take this drug again if you’ve ever had an allergic reaction to it. Taking it again could be fatal (cause death).
Warnings for people with certain health conditions
For people with kidney problems: Your kidneys clear this drug from your body. If you have kidney problems or a history of kidney disease, you may not be able to clear it from your body. This may increase the levels of the drug in your body and cause more side effects. This drug can also make your kidney function worse. To help prevent these problems, your doctor may prescribe a lower dosage of this drug for you.
To help prevent these problems, your doctor may prescribe a lower dosage of this drug for you.
For people with advanced HIV or a history of transplant: If you have advanced HIV or a history of bone marrow or kidney transplant, you may be at a higher risk of certain blood disorders. These conditions are called thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). They can result in severely low red blood cells and platelets in your body. TTP or HUS can cause death.
Warnings for other groups
For pregnant women: This drug is a category B pregnancy drug. That means two things:
- Research in animals has not shown a risk to the fetus when the mother takes the drug.
- There aren’t enough studies done in humans to show if the drug poses a risk to the fetus.
Talk to your doctor if you’re pregnant or planning to become pregnant. Animal studies do not always predict the way humans would respond. Therefore, this drug should only be used in pregnancy if clearly needed.
Call your doctor right away if you become pregnant while taking this drug.
For women who are breastfeeding: This drug may pass into breast milk and may cause side effects in a child who is breastfed. Talk to your doctor if you breastfeed your child. You may need to decide whether to stop breastfeeding or stop taking this medication.
For seniors: The kidneys of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
For children: This drug has not been studied for use in treatment or prevention of herpes simplex virus (HSV) infection in newborn babies. The following are other age limitations for use of this drug:
- Oral herpes (cold sores): This drug has been studied and approved for treatment of cold sores in children ages 12 years and older.

- Genital herpes: This drug has not been studied or approved for treatment of genital herpes in children younger than 18 years.
- Shingles: This drug has not been studied or approved for treatment of shingles in children younger than 18 years.
- Chickenpox: This drug has been studied and approved for treatment of chickenpox in children 2 to 18 years of age. This drug has not been studied or approved for treatment in children younger than 2 years of age.
All possible dosages and drug forms may not be included here. Your dosage, drug form, and how often you take the drug will depend on:
- your age
- the condition being treated
- the severity of your condition
- other medical conditions you have
- how you react to the first dose
Drug forms and strengths
Generic: Valacyclovir
- Form: oral tablet
- Strengths: 500 mg, 1 g
Brand: Valtrex
- Form: oral tablet
- Strengths: 500 mg, 1 g
Dosage for oral herpes
Adult dosage (ages 18–64 years)
- Typical dosage: 2 g, twice per day for 1 day, taken 12 hours apart.

- Note: Treatment should be started at the first sign of cold sore symptoms.
Child dosage (ages 12–17 years)
- Typical dosage: 2 g, twice per day for 1 day, taken 12 hours apart.
- Note: This drug should be started at the first sign of cold sore symptoms.
Child dosage (ages 0–11 years)
- This drug has not been studied or approved for treatment of oral herpes in children younger than 12 years.
Senior dosage (ages 65 years and older)
The kidneys of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
Your doctor may start you on a lowered dosage or a different treatment schedule. This can help keep levels of this drug from building up too much in your body.
Dosage for genital herpes
Adult dosage (ages 18–64 years)
- First episode: 1 g, taken twice per day for 10 days.
 This drug works best if it’s started within 48 hours of when the first symptom appears.
This drug works best if it’s started within 48 hours of when the first symptom appears. - Repeating episodes: 500 mg, taken twice per day for 3 days. Treatment should be started when the first symptom appears.
- For preventing flare-ups in people with a normal immune system: 500 mg to 1 g, taken once per day.
- For preventing flare-ups in people with HIV: 500 mg, taken twice per day.
- For reducing the risk of transmission to a sexual partner: 500 mg, taken once per day.
Child dosage (ages 0–17 years)
This drug has not been studied for the treatment of genital herpes in children younger than 18 years.
Senior dosage (ages 65 years and older)
The kidneys of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
This raises your risk of side effects.
Your doctor may start you on a lowered dosage or a different treatment schedule. This can help keep levels of this drug from building up too much in your body.
Dosage for shingles
Adult dosage (ages 18–64 years)
- Typical dosage: 1 g, taken three times per day for seven days.
- Note: Treatment should be started when the first symptom appears. This drug works best if it’s started within 48 hours of the first sign of a rash on the skin.
Child dosage (ages 0–17 years)
This drug has not been studied for the treatment of shingles in children younger than 18 years.
Senior dosage (ages 65 years and older)
The kidneys of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
Your doctor may start you on a lowered dosage or a different treatment schedule. This can help keep levels of this drug from building up too much in your body.
Dosage for chickenpox
Adult dosage (ages 18–64 years)
- Typical dosage: 1 g, taken 3 times per day for seven days.
- Note: Treatment should be started when the first symptom appears. This drug works best if it’s started within 48 hours of the first sign of a rash on the skin.
Child dosage (ages 2–18 years)
- Typical dosage: 20 mg per kilogram of the child’s body weight, taken 3 times per day for 5 days.
- Maximum dosage: 1 g, taken 3 times per day.
- Note: Treatment should be started at the earliest sign or symptom.
Child dosage (ages 0–1 year)
This drug has not been studied or approved for treatment of chickenpox in children younger than two years.
Senior dosage (ages 65 years and older)
The kidneys of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
Your doctor may start you on a lowered dosage or a different treatment schedule. This can help keep levels of this drug from building up too much in your body.
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs affect each person differently, we cannot guarantee that this list includes all possible dosages. This information is not a substitute for medical advice. Always speak with your doctor or pharmacist about dosages that are right for you.
Valacyclovir oral tablet is used for short-term treatment of oral herpes, genital herpes, shingles, or chickenpox. It’s used for long-term treatment to prevent genital herpes, and to treat genital herpes that recurs (comes back).
This drug comes with serious risks if you don’t take it as prescribed.
If you stop taking the drug suddenly or don’t take it at all: The symptoms of your viral infection may not get better, or may get worse.
If you miss doses or don’t take the drug on schedule: Your medication may not work as well or may stop working completely. If you’re taking this drug to prevent flare-ups of the infection, a certain amount needs to be in your body at all times. You should not stop taking this drug unless your doctor tells you to stop.
If you take too much: You could have dangerous levels of the drug in your body. Symptoms of an overdose of this drug can include more severe side effects, such as:
- headache
- nausea
- tiredness
- dizziness
- diarrhea
- constipation
- weakness or lack of energy
If you think you’ve taken too much of this drug, call your doctor or seek guidance from the American Association of Poison Control Centers at 1-800-222-1222 or through their online tool. But if your symptoms are severe, call 911 or go to the nearest emergency room right away.
But if your symptoms are severe, call 911 or go to the nearest emergency room right away.
What to do if you miss a dose: Take your dose as soon as you remember. But if you remember just a few hours before your next scheduled dose, take only one dose. Never try to catch up by taking two doses at once. This could result in dangerous side effects.
How to tell if the drug is working: Your symptoms from the viral infection should improve.
Keep these considerations in mind if your doctor prescribes valacyclovir for you.
General
- You can take this drug with or without food. Taking it with food may help reduce any upset stomach.
- Take this drug at the time(s) recommended by your doctor.
Storage
- Store valacyclovir at room temperature between 59°F and 77°F (15°C and 25°C).
- Keep this drug away from light.
- Don’t store this medication in moist or damp areas, such as bathrooms.

Refills
A prescription for this medication is refillable. You should not need a new prescription for this medication to be refilled. Your doctor will write the number of refills authorized on your prescription.
Travel
When traveling with your medication:
- Always carry your medication with you. When flying, never put it into a checked bag. Keep it in your carry-on bag.
- Don’t worry about airport X-ray machines. They can’t harm your medication.
- You may need to show airport staff the pharmacy label for your medication. Always carry the original prescription-labeled container with you.
- Don’t put this medication in your car’s glove compartment or leave it in the car. Be sure to avoid doing this when the weather is very hot or very cold.
Availability
Not every pharmacy stocks this drug. When filling your prescription, be sure to call ahead to make sure your pharmacy carries it.
Hidden costs
You may need to have blood tests during your treatment with this drug. The cost of these tests will depend on your insurance coverage.
The cost of these tests will depend on your insurance coverage.
Prior authorization
Many insurance companies require a prior authorization for this drug. This means your doctor will need to get approval from your insurance company before your insurance company will pay for the prescription.
There are other drugs available to treat your condition. Some may be better suited for you than others. Talk to your doctor about other drug options that may work for you.
Disclaimer: Healthline has made every effort to make certain that all information is factually correct, comprehensive, and up-to-date. However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or other healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
Guidelines for the Treatment of Genital Herpes uMEDp
Genital herpes is a sexually transmitted disease that causes recurrent genital lesions. The causative agents are herpes simplex viruses, predominantly type II, although the etiological role of type I virus has increased significantly over the past two decades.
Table. Doses of drugs for the treatment of genital herpes
Despite the severe clinical manifestations of the primary infection and potentially dangerous complications leading to hospitalization and death, genital herpes has received insufficient attention in the non-specialized medical literature. Meanwhile, the spread of the disease has become epidemic, which is associated with a large number of undiagnosed and untreated cases and a high frequency of asymptomatic and subclinical course (2). Symptomatic genital herpes affects 86 million people worldwide. The total number of people infected with the virus cannot be determined, but it is assumed that, for example, in the United States, every fourth to sixth inhabitant of the virus is a carrier (3-4). nine0003
Symptomatic genital herpes affects 86 million people worldwide. The total number of people infected with the virus cannot be determined, but it is assumed that, for example, in the United States, every fourth to sixth inhabitant of the virus is a carrier (3-4). nine0003
The main means for the treatment of genital herpes are antiviral drugs from the group of nucleoside analogues. The most widely used drug continues to be acyclovir, which appeared on the world pharmaceutical market about 20 years ago. Being an analogue of nucleosides, the drug undergoes phosphorylation under the influence of viral thymidine kinase, and then, with the help of host cell enzymes, it turns into di- and triphosphate. The latter acts as a substrate for viral DNA polymerase, thus leading to disruption of DNA virus replication. nine0003
The effectiveness of acyclovir for the treatment of genital herpes has been shown in numerous clinical studies. When taken orally, the drug is well tolerated by patients and has an excellent benefit/risk ratio.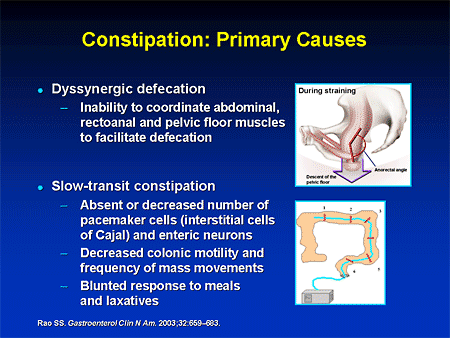 Side effects develop in less than 10% of patients and are usually limited to nausea, vomiting and headache (5).
Side effects develop in less than 10% of patients and are usually limited to nausea, vomiting and headache (5).
The main disadvantages of acyclovir include low bioavailability (15-20%) and a short half-life from tissues (0.7 h) and blood plasma (2.7 h) (5). To maintain therapeutic concentrations in the body, the drug must be taken up to 5 times a day, which negatively affects the accuracy of the treatment regimen by patients and may lead to a decrease in the effectiveness of therapy. nine0003
The pharmacokinetic shortcomings of acyclovir were largely overcome in the development of valaciclovir, a prodrug metabolized in the intestinal wall and liver to form acyclovir. Valaciclovir has a significantly higher oral bioavailability (54%) and provides higher and longer serum concentrations of acyclovir (6-8), which allows it to be taken twice a day. With repeated oral administration of valaciclovir in high doses (4-8 g/day), the concentrations of the active substance in the blood are comparable to the concentrations created by intravenous administration of acyclovir (5-10 mg/kg 3 times/day) (8, 9).
The kinetics of acyclovir released from valacyclovir is similar to that of acyclovir preparations in healthy volunteers, patients with kidney disease, HIV infection (10), elderly patients and senile volunteers, both receiving and not receiving concomitant diuretic therapy (11).
The safety profile of valacyclovir does not differ significantly from that of acyclovir. Better absorption results in fewer gastrointestinal adverse reactions (5). Thrombotic microangiopathy has been described in patients with AIDS and other immune disorders when using valaciclovir in high doses (8 g/day), but the causal relationship between the drug and this complication has not been conclusively confirmed (12). In patients receiving high doses of valaciclovir after kidney transplantation, thrombotic microangiopathy was not observed (13). In addition, for the treatment of genital herpes, the drug is used in much lower doses. nine0003
The third analogue of nucleosides, famciclovir, like valaciclovir, is a prodrug that is converted in the intestinal wall and in the liver into penciclovir. Penciclovir has a similar mechanism and spectrum of antiviral action to acyclovir, however, due to low bioavailability, it can only be used topically (5). With oral famciclovir, the bioavailability of penciclovir is 77% (14). Compared to acyclovir, penciclovir has a significantly longer half-life from tissues (10-20 hours) (15). Intracellular concentrations of penciclovir triphosphate exceed those of acyclovir triphosphate by about 30 times (16). However, herpes simplex virus DNA polymerase has a greater affinity for acyclovir triphosphate than for penciclovir triphosphate. Thus, the differences in mechanism of action between penciclovir and aciclovir are predominantly quantitative and tend to balance each other out (16). nine0003
Penciclovir has a similar mechanism and spectrum of antiviral action to acyclovir, however, due to low bioavailability, it can only be used topically (5). With oral famciclovir, the bioavailability of penciclovir is 77% (14). Compared to acyclovir, penciclovir has a significantly longer half-life from tissues (10-20 hours) (15). Intracellular concentrations of penciclovir triphosphate exceed those of acyclovir triphosphate by about 30 times (16). However, herpes simplex virus DNA polymerase has a greater affinity for acyclovir triphosphate than for penciclovir triphosphate. Thus, the differences in mechanism of action between penciclovir and aciclovir are predominantly quantitative and tend to balance each other out (16). nine0003
Aciclovir and penciclovir do not differ in their activity against herpes simplex virus in cell culture, however, after acyclovir is discontinued, virus replication resumes much faster than after penciclovir is discontinued (13). Famciclovir is superior to valaciclovir in its ability to eliminate "competent latency", i. e., the state in which the virus is able to reactivate and cause relapses of the disease (18, 19). In an observational study in patients treated with famciclovir (250 mg 3 times / day for 5 days), relapses within 1-6 months after the first episode developed less frequently than in patients treated with acyclovir (200 mg 5 times / day . within 5 days) (20). nine0003
e., the state in which the virus is able to reactivate and cause relapses of the disease (18, 19). In an observational study in patients treated with famciclovir (250 mg 3 times / day for 5 days), relapses within 1-6 months after the first episode developed less frequently than in patients treated with acyclovir (200 mg 5 times / day . within 5 days) (20). nine0003
Famciclovir has an excellent safety profile. A tolerability analysis based on the results of 13 clinical studies showed that the adverse reaction profile of famciclovir in patients with genital herpes (791 patients) did not differ from placebo (21). In studies on healthy male volunteers, no clinically significant pharmacokinetic interactions of famciclovir with allopurinol, digoxin, cimetidine, zidovudine, or theophylline have been identified (22). The bioavailability of penciclovir, which is formed during the metabolism of famciclovir, does not depend on food intake (23). Its pharmacokinetics do not differ between young and old people (24). When taken for 4–12 months in healthy male volunteers, the drug did not adversely affect sperm (25). nine0003
When taken for 4–12 months in healthy male volunteers, the drug did not adversely affect sperm (25). nine0003
Aciclovir, valaciclovir and famciclovir show comparable clinical efficacy in reducing the duration and severity of genital herpes episodes and reducing virus shedding (26-28). However, they have no effect on dormant viruses.
Treatment first episode genital herpes
The first episode of primary genital herpes is considered the clinical manifestation of the disease in patients without antibodies to herpes simplex viruses type I or II. The first episode is characterized by the most severe course. As a rule, in addition to local lesions that persist for 2-3 weeks, systemic symptoms and regional adenopathy are observed. A small number of patients, more often immunocompromised, develop viral meningitis. nine0003
Symptomatic therapy of the first episode consists in the appointment of analgesics. Opioid drugs should be avoided as they cause constipation. Nucleoside analogues are used for etiotropic treatment.
Opioid drugs should be avoided as they cause constipation. Nucleoside analogues are used for etiotropic treatment.
Efficacy of oral acyclovir at a dose of 200 mg 5 times / day.
within 5-10 days is indicated in double-blind, placebo-controlled clinical trials (29, 30). In patients treated with acyclovir, the virus shedding period, crusting time, and healing time were significantly shortened. An increase in the oral daily dose of the drug to 4 g did not lead to an increase in the effectiveness of therapy (31). In addition, gastrointestinal side effects (8%) were significantly more common with the high dose than with the standard dose (0%). nine0003
In order to improve patient adherence to treatment, the US Centers for Disease Control and Prevention (CDC) recommends the use of acyclovir 400 mg 3 times a day, but this regimen is not approved by the FDA. Topical acyclovir cream is not effective (6). The addition of cream to oral therapy also did not improve clinical outcomes (32). In severe cases with neurological complications, acyclovir is recommended to be administered intravenously at 5-10 mg/kg 3 times a day (5). nine0003
In severe cases with neurological complications, acyclovir is recommended to be administered intravenously at 5-10 mg/kg 3 times a day (5). nine0003
Valaciclovir at a dose of 1 g 2 times / day. shows equal efficacy with acyclovir at a dose of 200 mg 5 times / day. in the first episode of genital herpes in immunocompetent patients (33). The tolerability of valacyclovir is similar to that of acyclovir (33). In different countries, valaciclovir is approved for the treatment of the first episode of genital herpes at a dose of 500 mg or 1 g 2 times a day. within 10 days (25).
Famciclovir has been studied in comparative clinical trials with acyclovir (200 mg 5 times a day) at doses of 125, 250 and 500 mg 3 times a day (27, 28). None of the studies showed significant differences between the comparison groups. For the treatment of genital herpes, famciclovir is recommended to prescribe 250 mg 3 times / day. within 5 days, with severe infection - 10 days (25). nine0003
Episodic treatment relapses genital virus
In clinical studies, with the use of acyclovir at a standard daily dose (200 mg 5 times / day) for the episodic treatment of relapses of genital herpes, a shortening of the period of virus shedding, crusting time and healing time was noted. The duration of symptoms and the time of occurrence of new relapses did not change under the influence of the drug (28, 34). The guidelines for the management of genital herpes, issued in 1998. The US Centers for Disease Control and Prevention (CDC) suggested the use of acyclovir in doses of 400 mg 3 times / day. or 800 mg 2 times / day. within 5 days. However, these treatment regimens have not been studied in adequate clinical trials.
The duration of symptoms and the time of occurrence of new relapses did not change under the influence of the drug (28, 34). The guidelines for the management of genital herpes, issued in 1998. The US Centers for Disease Control and Prevention (CDC) suggested the use of acyclovir in doses of 400 mg 3 times / day. or 800 mg 2 times / day. within 5 days. However, these treatment regimens have not been studied in adequate clinical trials.
When applied topically in the form of a 5% polyethylene glycol ointment, acyclovir resulted in a decrease in the period of virus shedding, but did not cause clinical improvement (6).
Valaciclovir at a dose of 500 or 1000 mg 2 times / day. for 5 days, administered on the first day after the onset of symptoms, significantly reduced the period of virus shedding and accelerated the healing of lesions compared with placebo (35). Tolerability of the drug did not differ from that of placebo. One study showed that fewer patients (10%) developed vesiculo-ulcerative lesions with valaciclovir than with placebo (25).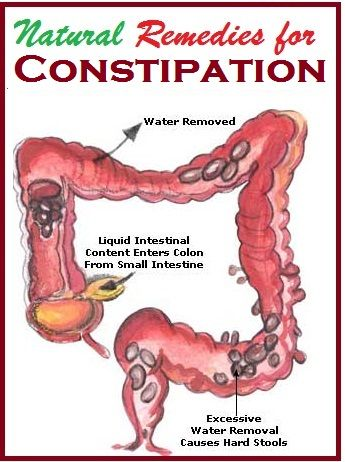 The results of another study suggest, based on the natural period of virus shedding, that a 3-day course of treatment with valaciclovir is as effective as a 5-day course (36). In July 2001, the FDA approved a 3-day course of treatment for recurrences of genital herpes with valaciclovir (500 mg 2 times a day). Comparative studies with acyclovir showed equal clinical efficacy of both drugs (37). An important advantage of valaciclovir is a more convenient mode of application. nine0003
The results of another study suggest, based on the natural period of virus shedding, that a 3-day course of treatment with valaciclovir is as effective as a 5-day course (36). In July 2001, the FDA approved a 3-day course of treatment for recurrences of genital herpes with valaciclovir (500 mg 2 times a day). Comparative studies with acyclovir showed equal clinical efficacy of both drugs (37). An important advantage of valaciclovir is a more convenient mode of application. nine0003
Famciclovir at doses of 125, 250, and 500 mg twice daily given within the first 6 hours of symptom onset resulted in a reduction in healing time, viral shedding time, and duration of edema of the lesions compared with placebo (38). When it was used, pain, burning, tingling, and soreness when touched were significantly reduced. The side effects of famciclovir did not differ from those of placebo. Famciclovir is recommended for the episodic treatment of recurrences of genital herpes at a dose of 125 mg 2 times a day. within 5 days. The advantages of famciclovir, as well as valaciclovir, are in a more convenient treatment regimen compared to acyclovir. nine0003
within 5 days. The advantages of famciclovir, as well as valaciclovir, are in a more convenient treatment regimen compared to acyclovir. nine0003
The effectiveness of episodic treatment of recurrences of genital herpes depends on the time of the start of taking the drugs. A Canadian study showed that when famciclovir was started within the first 6 hours after the onset of prodromal symptoms or the first genital lesions, there was a significant reduction in virus shedding and faster healing of the lesions. Moreover, taking the drug before the onset of virus shedding increased the likelihood of preventing virus shedding during a relapse (39). Relapse treatment should be started as soon as possible, so patients should always have a supply of the drug.
Ongoing suppressive therapy
Patients with frequent (more than 6-8 per year) and severe relapses, psychological trauma or disruption of a normal lifestyle caused by the disease are shown to continue suppressive therapy. For this purpose, acyclovir 400 mg 2 times / day is used for a long time (within 6-12 months); valaciclovir 500 mg 1 time / day. (with a number of relapses of 10 or less per year) or 1000 mg 1 time / day. (with the number of relapses more than 10 per year) or famciclovir 250 mg 2 times / day. Daily aciclovir has been shown to reduce relapse rates by 80%, with 25-30% of patients not relapsing (40). Similar results were obtained in studies with valaciclovir and famciclovir. When using famciclovir at a daily dose of 500 mg, divided into 2 doses, for 16 weeks, relapses did not develop during the treatment period in 78% of patients (41), when using valaciclovir at the same dose - in 69% of patients (33). All three drugs have proven to be effective agents for long-term suppressive therapy of recurrent genital herpes. Apparently, the cost of the annual dose and the convenience of the regimen for the patient should be the decisive factors in choosing a particular drug.
For this purpose, acyclovir 400 mg 2 times / day is used for a long time (within 6-12 months); valaciclovir 500 mg 1 time / day. (with a number of relapses of 10 or less per year) or 1000 mg 1 time / day. (with the number of relapses more than 10 per year) or famciclovir 250 mg 2 times / day. Daily aciclovir has been shown to reduce relapse rates by 80%, with 25-30% of patients not relapsing (40). Similar results were obtained in studies with valaciclovir and famciclovir. When using famciclovir at a daily dose of 500 mg, divided into 2 doses, for 16 weeks, relapses did not develop during the treatment period in 78% of patients (41), when using valaciclovir at the same dose - in 69% of patients (33). All three drugs have proven to be effective agents for long-term suppressive therapy of recurrent genital herpes. Apparently, the cost of the annual dose and the convenience of the regimen for the patient should be the decisive factors in choosing a particular drug.
Suppressive therapy is well tolerated. It has been shown to be safe with daily use for 5 years (42). However, before prescribing suppressive therapy, it is recommended to conduct hematological and biochemical studies and determine the state of kidney function. During the treatment period, patients should be under medical supervision, women are advised to avoid pregnancy. nine0003
It has been shown to be safe with daily use for 5 years (42). However, before prescribing suppressive therapy, it is recommended to conduct hematological and biochemical studies and determine the state of kidney function. During the treatment period, patients should be under medical supervision, women are advised to avoid pregnancy. nine0003
Daily suppressive therapy leads to a significant reduction in asymptomatic viral shedding, but does not completely eliminate it. The patient should be warned when deciding on its appointment. As an alternative, the patient should be offered selective prophylaxis (for example, during periods of stress). To date, a reduction in the risk of infection transmission under the influence of suppressive therapy has not been proven.
Regardless of whether suppressive treatment is given or not, the recurrence rate decreases on average 7 years after the first episode, so lifelong treatment is usually not needed. nine0003
Treatment of genital herpes in pregnant women
Women suffering from genital herpes during pregnancy have an increased risk of spontaneous abortions and the birth of children with low weight.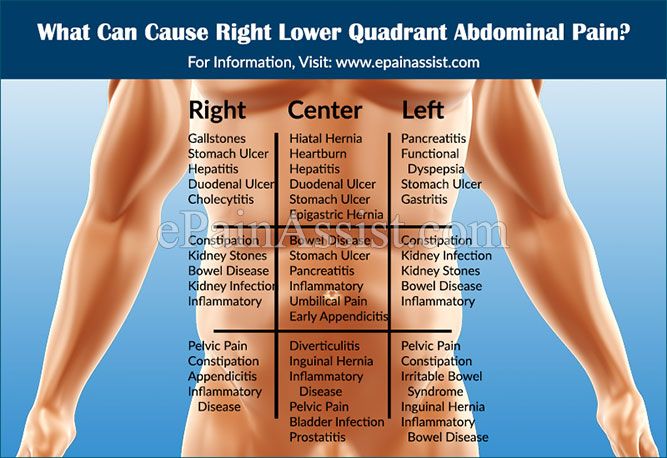 In rare cases, a congenital infection may develop in the fetus. The highest risk of infection is during the passage of the child through the birth canal. The risk of transmission has been estimated at 50% for primary maternal herpes during delivery and 0–3% for recurrent herpes (43). Most often, infection occurs from asymptomatic mothers (44). nine0003
In rare cases, a congenital infection may develop in the fetus. The highest risk of infection is during the passage of the child through the birth canal. The risk of transmission has been estimated at 50% for primary maternal herpes during delivery and 0–3% for recurrent herpes (43). Most often, infection occurs from asymptomatic mothers (44). nine0003
In newborns, the disease occurs in three main forms: localized, with damage to the central nervous system, and disseminated. Mortality is 15% for infections involving the central nervous system and 57% for disseminated infection (45).
In order to reduce the risk of subclinical viral shedding and relapse episodes during labor, many clinicians recommend that pregnant women receive suppressive antiviral therapy. Nucleoside analogues are not officially approved for use during pregnancy, but many studies have shown the safety of acyclovir (46, 47). Valaciclovir and famciclovir, according to the FDA classification, belong to group B, i. e. to drugs for which no teratogenic effect has been detected in experiments on animals, and adequate clinical trials in humans have not been conducted. Studies are currently underway to determine the efficacy and safety of valaciclovir during pregnancy. nine0003
e. to drugs for which no teratogenic effect has been detected in experiments on animals, and adequate clinical trials in humans have not been conducted. Studies are currently underway to determine the efficacy and safety of valaciclovir during pregnancy. nine0003
Data on the effectiveness of suppressive therapy in preventing infection in the fetus are extremely limited. In a randomized trial including 46 women with a first episode of genital herpes, it was not possible to determine the effectiveness of treatment with acyclovir started on
36 weeks of gestation, as none of the newborns developed an infection. However, in the group of patients receiving suppressive therapy, a significant decrease in the frequency of caesarean section was noted (48). In another placebo-controlled study that included 150 women with a history of genital herpes (49), it has been shown that acyclovir (200 mg 3 times / day), taken from the 38th week of pregnancy, is able to prevent recurrence of the disease.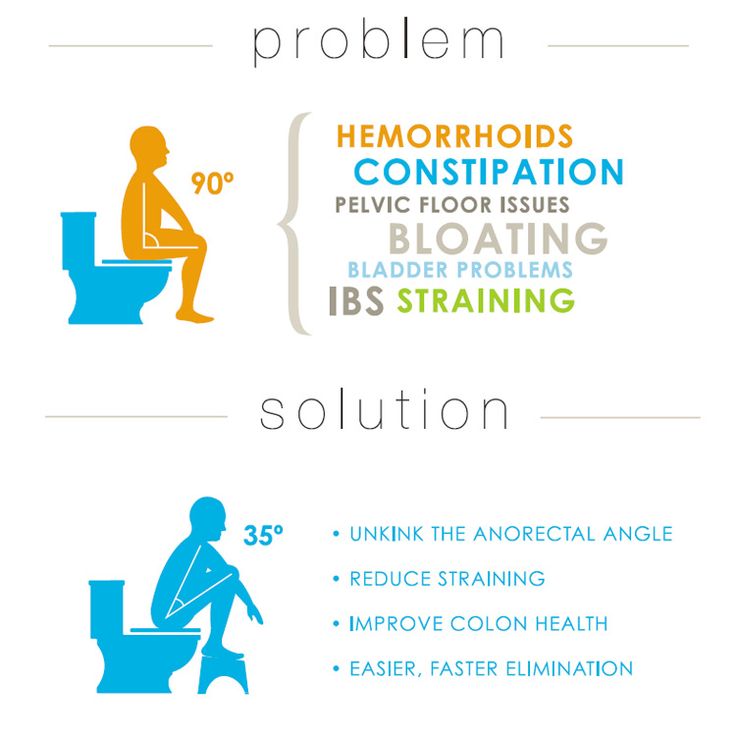 In patients treated with acyclovir, no relapse occurred in any case, while in the control group there were 33 relapses, 21 of them during childbirth.
In patients treated with acyclovir, no relapse occurred in any case, while in the control group there were 33 relapses, 21 of them during childbirth.
Treatment of genital herpes , caused by aciclovir - resistant strains
Long-term treatment with nucleoside analogs is generally not associated with the risk of developing viral resistance. In immunocompetent patients receiving suppressive therapy, acyclovir-resistant strains of the herpes simplex virus were isolated, but no correlation was observed between in vitro resistance and the therapeutic efficacy of the drug (25). The most common mechanism for the development of resistance is a gene mutation that leads to impaired thymidine kinase production (50), so aciclovir-resistant strains usually show cross-resistance to penciclovir. Much less common are mutations that lead to dysfunction of thymidine kinase and DNA polymerase (50, 51). In these cases, selective resistance to one of the drugs is possible. Mathematical methods of analysis suggest that decades are needed to change the current model of virus resistance (52). nine0003
In these cases, selective resistance to one of the drugs is possible. Mathematical methods of analysis suggest that decades are needed to change the current model of virus resistance (52). nine0003
In immunocompromised patients with long-term use of antiviral drugs, resistance develops in about 5% of cases and can lead to treatment failure (53, 54). Clinically significant resistance has also been described in patients undergoing bone marrow transplantation (55).
Currently, foscarnet is considered as the drug of choice for the treatment of acyclovir-resistant infections caused by herpes simplex virus (56). It belongs to non-competitive inhibitors of viral DNA polymerase. The drug blocks the receptors for binding to viral DNA pyrophosphate and disrupts the elongation of its chain. Unlike acyclovir and penciclovir, it does not require thymidine kinase-mediated phosphorylation and is active against acyclovir-resistant thymidine kinase-deficient strains (56). nine0003
Foscarnet has a low oral bioavailability, so it is administered intravenously and topically.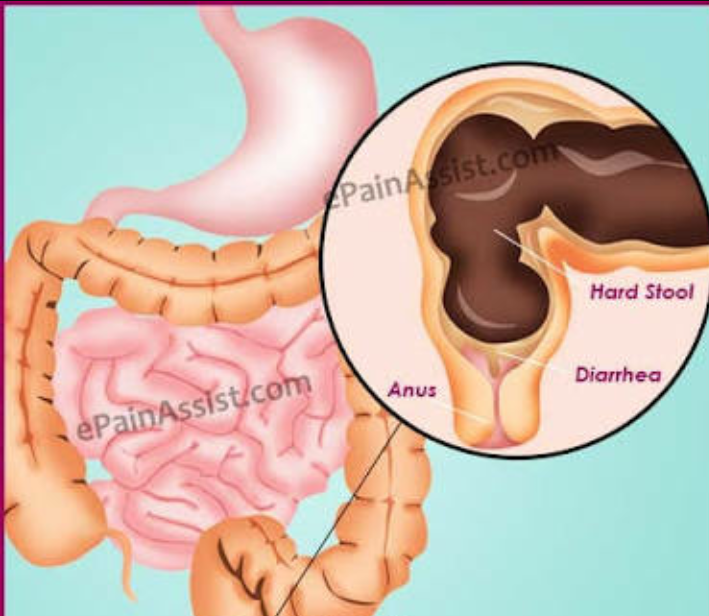 The effectiveness of intravenous foscarnet in genital herpes caused by acyclovir-resistant strains has been shown in several clinical studies. In an uncontrolled study, the drug was effective in 81% of patients with acyclovir-resistant genital herpes that developed on the background of HIV infection (57). In another study in patients with HIV infection, foscarnet was superior to vidarabine in terms of the healing time of herpes mucocutaneous lesions caused by acyclovir-resistant strains and the period of cessation of virus shedding (58). When applied topically, the drug did not show sufficient effectiveness (25). nine0003
The effectiveness of intravenous foscarnet in genital herpes caused by acyclovir-resistant strains has been shown in several clinical studies. In an uncontrolled study, the drug was effective in 81% of patients with acyclovir-resistant genital herpes that developed on the background of HIV infection (57). In another study in patients with HIV infection, foscarnet was superior to vidarabine in terms of the healing time of herpes mucocutaneous lesions caused by acyclovir-resistant strains and the period of cessation of virus shedding (58). When applied topically, the drug did not show sufficient effectiveness (25). nine0003
Foscarnet is potentially toxic. Side effects include impaired kidney function, gastrointestinal disturbances, magnesium and calcium metabolism disorders, anemia, genital ulceration, and seizures (59). To prevent serious side effects, treatment should be carried out under close medical supervision, maintain an adequate level of hydration, and avoid the simultaneous administration of pentamidine.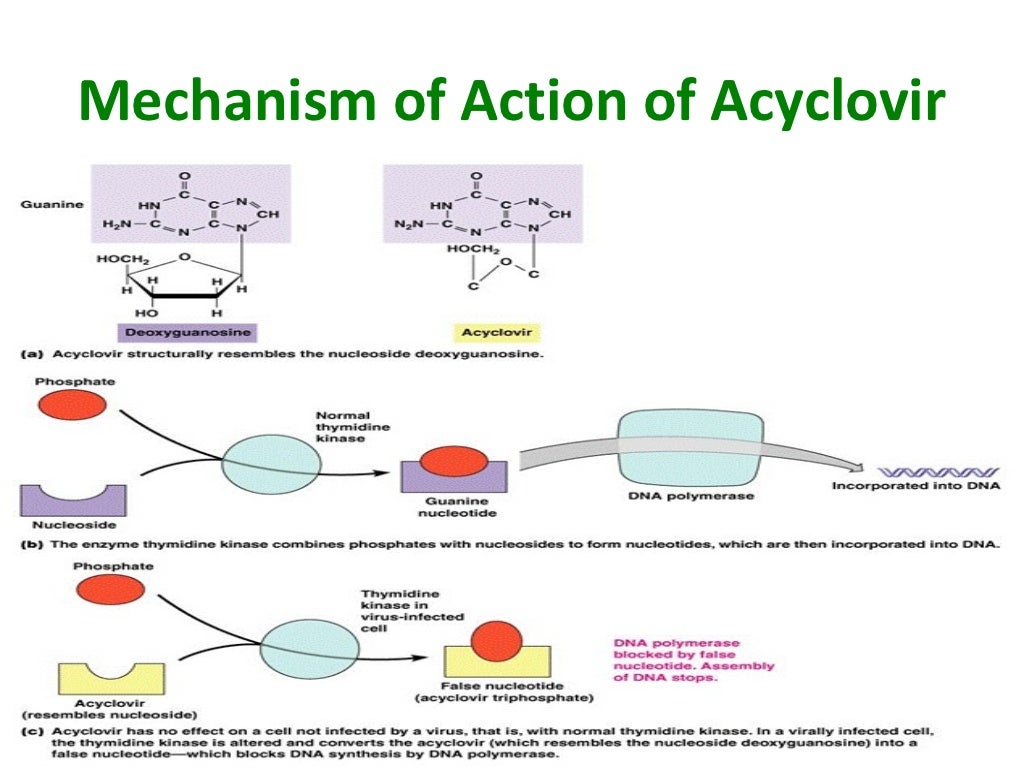
The use of foscarnet limits the intravenous route of administration. It can develop resistance due to mutation of DNA polymerase (60). Clinical strains of herpes simplex virus have been described that are simultaneously resistant to acyclovir and foscarnet (61). nine0003
Cidofovir shows pronounced activity against herpes simplex virus in vitro and in vivo (62, 63). It is an acyclic analog of phosphonate and has a wide spectrum of antiviral activity. The drug undergoes phosphorylation under the influence of cellular enzymes into active diphosphate, bypassing the first stage of phosphorylation with the participation of virus enzymes, which is necessary for acyclovir and penciclovir (64). Cidofovir has a low oral bioavailability (less than 5%), which allows it to be used only topically. The advantage is a long half-life from the cell. Intracellular concentrations of cidofovir mono- and diphosphate are maintained for 24 and 65 hours, respectively (65). nine0003
The effectiveness of a single dose of the drug compared with placebo has been shown in immunocompetent patients with recurrent genital herpes (66). In this study, 12 hours after the onset of the first lesions, patients received cidofovir gel at a concentration of 1.3 or 5% or placebo. Local toxic reactions of the drug slowed down the healing of lesions in a number of patients. They were dose-dependent and were observed in three of 23 patients treated with 5% gel and in 1 of 21 patients treated with 3% gel. Further studies are needed to determine the maximum gel concentration well tolerated by patients. nine0003
In this study, 12 hours after the onset of the first lesions, patients received cidofovir gel at a concentration of 1.3 or 5% or placebo. Local toxic reactions of the drug slowed down the healing of lesions in a number of patients. They were dose-dependent and were observed in three of 23 patients treated with 5% gel and in 1 of 21 patients treated with 3% gel. Further studies are needed to determine the maximum gel concentration well tolerated by patients. nine0003
In a clinical placebo-controlled study, cidofovir gel applied once a day for 5 days showed a pronounced virological and clinical effect in patients with acyclovir-resistant genital herpes on the background of HIV infection (67). Complete resolution of clinical symptoms was observed in 27% of patients treated with cidofovir 0.3%, in 33% of patients treated with cidofovir 1%, and in 0% of patients treated with placebo. The median reduction in the area of damage was 58% in patients treated with cidofovir compared with 0% in the control group. Unfortunately, the small number of patients who participated in this study does not allow us to assess the statistical significance of the results obtained. nine0003
Unfortunately, the small number of patients who participated in this study does not allow us to assess the statistical significance of the results obtained. nine0003
In a clinical study in bone marrow transplant recipients, cidofovir was effective in treating genital herpes caused by virus strains resistant to acyclovir and foscarnet (61).
There is experience with the use of tifluridine ophthalmic solution for the treatment of genital herpes caused by acyclovir-resistant strains (). Tifluridine is a pyrimidine nucleoside analog that, like cidophyr, acts independently of viral thymidine kinase. High toxicity does not allow the drug to be taken orally. Small clinical studies have shown a beneficial effect of tifluridine in acyclovir-resistant genital herpes in patients with HIV infection (68). In infections caused by acyclovir- or acyclovir/foscarnet-resistant herpes simplex viruses, tifluridine showed a synergistic effect with interferon-a (69). However, to determine the therapeutic value of tifluridine in genital herpes, further study is needed in large controlled studies.
Several studies examining the efficacy of topical interferon formulations for the treatment of genital herpes have yielded conflicting results. Local application of interferon α-2a 6 times / day. in the form of an aqueous solution proved to be ineffective for the treatment of herpes genital lesions (70). In a placebo-controlled study that included 387 patients, on the contrary, the effectiveness of interferon α-2a gel was shown when applied 4 times / day. within 4 days in case of recurrence of genital herpes (71). In patients of both sexes, the period of virus shedding decreased, in addition, in men, there was a significant decrease in pain, itching, and crusting time. In a study including 25 patients, a beneficial effect of interferon ß was shown (72). nine0003
In Germany and Canada, edoxudine, an analogue of deoxythymidine, has been approved for the treatment of genital herpes, showing pronounced antiherpes activity in vitro and in vivo (73, 74). The mechanism of action of the drug is similar to that of acyclovir. When applied topically, it is better absorbed than polyethylene glycol-based acyclovir ointment. In a multicentre, placebo-controlled study, edoxudin 3% cream applied for 5 days significantly reduced viral shedding in both sexes (75). In women, there was also a decrease in soreness and adenopathy in the groin. nine0003
When applied topically, it is better absorbed than polyethylene glycol-based acyclovir ointment. In a multicentre, placebo-controlled study, edoxudin 3% cream applied for 5 days significantly reduced viral shedding in both sexes (75). In women, there was also a decrease in soreness and adenopathy in the groin. nine0003
A dosage form of acyclovir with controlled release of the active substance has been developed (76). Its advantage is the prolongation of the half-life of acyclovir. Clinical trials conducted in Europe show that long-acting acyclovir is equally effective as short-acting acyclovir. It has been suggested that the use of a drug with an extended half-life may lead to a decrease in the number of relapses during suppressive therapy, but it requires further study. nine0003
In experiments on guinea pigs and in a clinical study, the beneficial effect of resiquimod, an immune response modifier from the imidazoquinoline group, has been shown (77, 78). In patients with genital herpes, when topical treatment was started within a day after the onset of symptoms, there was an increase in the time before a relapse occurred. In 52 immunocompetent patients with a history of at least 6 relapses per year, the median time to the first relapse was 169days with resiquimod and 57 days with placebo (79). The drug is currently undergoing phase III clinical trials.
In 52 immunocompetent patients with a history of at least 6 relapses per year, the median time to the first relapse was 169days with resiquimod and 57 days with placebo (79). The drug is currently undergoing phase III clinical trials.
Despite advances in the treatment of genital herpes over the past few years, the epidemic of the disease continues to spread throughout the world. The main drugs for the treatment of infection are nucleoside analogues. The emergence of generic acyclovir preparations has significantly reduced the cost of treatment. New drugs from this group - the prodrugs valaciclovir and famciclovir - have better pharmacokinetic properties compared to acyclovir, which allows to increase the degree of patient compliance with the treatment regimen. In the treatment of the first episode of genital herpes, the drugs show comparable clinical efficacy. nine0003
Direct comparative studies between the three nucleoside analogues in the episodic treatment of relapses of genital herpes have not been conducted. Apparently, the selection of an antiviral agent should be individual. All three drugs should be tried (not simultaneously) to determine patient preference.
Apparently, the selection of an antiviral agent should be individual. All three drugs should be tried (not simultaneously) to determine patient preference.
Episodic treatment is not suitable for all patients. Due to good efficacy and safety, suppressive therapy is now becoming more widespread. It has been suggested that suppressive therapy, by reducing asymptomatic shedding of the virus, can stop the spread of infection, but to date it has not been proven. Acyclovir has been successfully used for suppressive therapy. It is not inferior in effectiveness to valaciclovir. A single dose of valaciclovir per day is convenient for patients with a rare relapse rate. Comparative studies of acyclovir and famciclovir have not been conducted. nine0003
Relapses of the disease can also develop against the background of suppressive therapy, which is associated with a short half-life of antiviral agents. With the abolition of suppressive therapy, the risk of infection transmission may increase.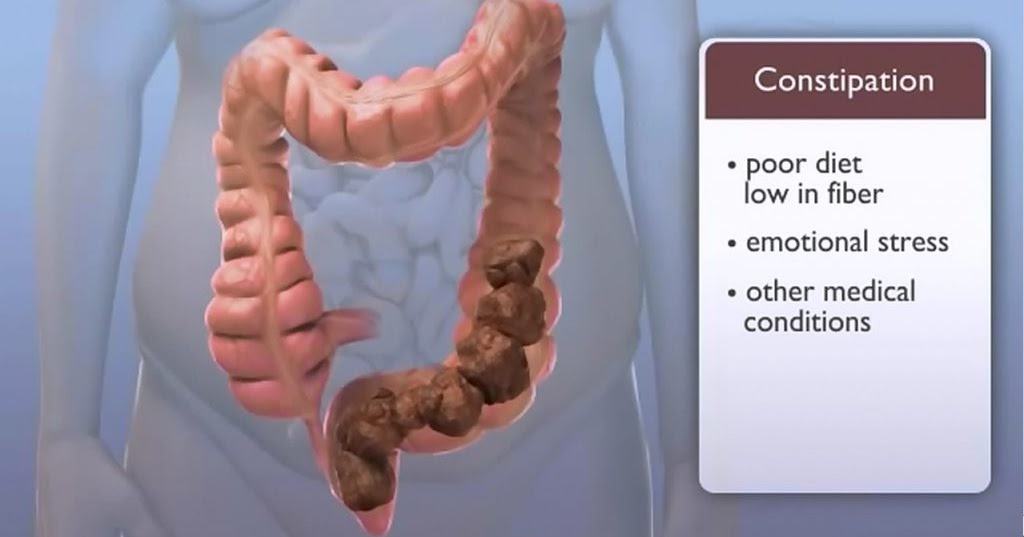 This question needs to be studied in special clinical studies. Until the results of these studies are available, suppressive therapy should be discontinued cautiously, counseling the patient about the safety of sexual behavior.
This question needs to be studied in special clinical studies. Until the results of these studies are available, suppressive therapy should be discontinued cautiously, counseling the patient about the safety of sexual behavior.
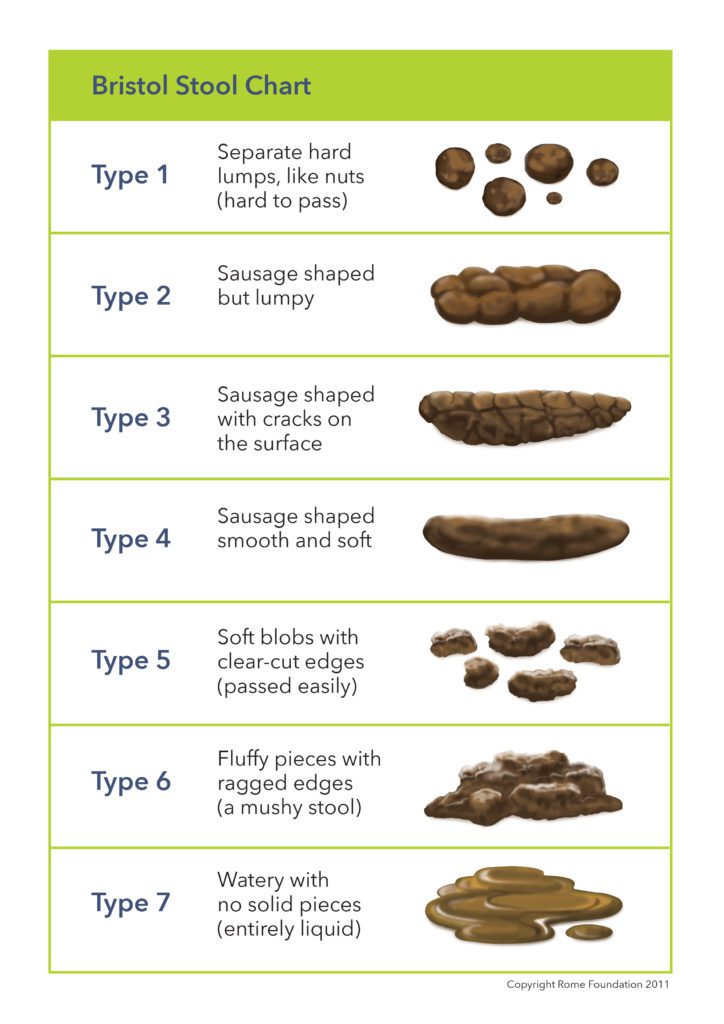
How to deal with constipation after taking antibiotics: how to restore bowel function
How do antibiotics affect bowel function?
The main mechanism by which antibiotics cause disruption of the normal functioning of the intestine is not associated with the toxic effect of drugs, but with their effect on the intestinal microflora 2 .
Antibacterial drugs significantly inhibit the growth of not only pathogenic microorganisms, but also the normal microflora in the large intestine, which is directly involved in the digestive process.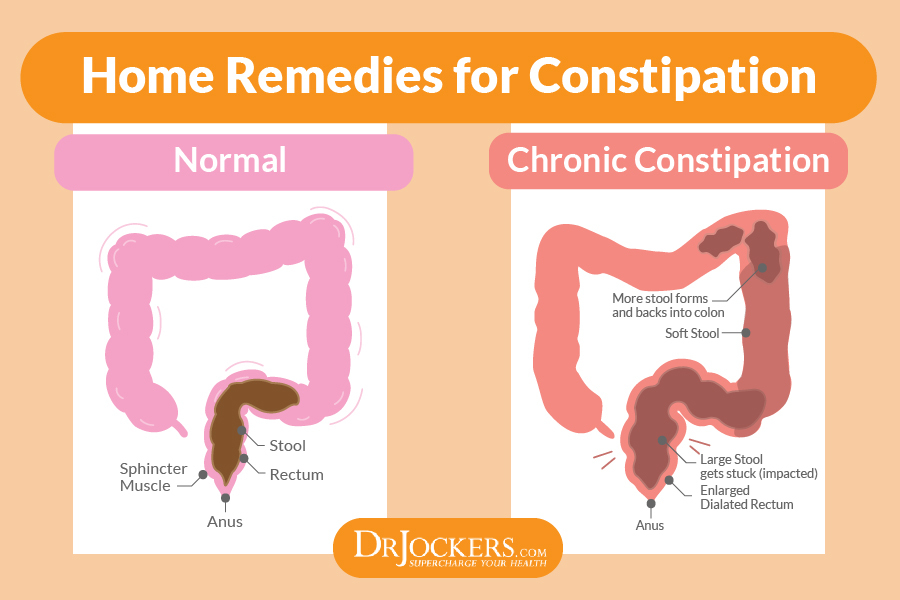 As a result, quantitative and qualitative changes in the composition of the intestinal microflora, which may be accompanied by a disorder of the gastrointestinal tract 3 . Constipation is one of the possible manifestations of dysbacteriosis and microflora disorders associated with taking antibiotics. In this case, as shown by a number of studies, there is a death of normal intestinal microflora, which includes bacteroids, bifidobacteria and lactobacilli, as well as other bacteria useful to us 4 .
As a result, quantitative and qualitative changes in the composition of the intestinal microflora, which may be accompanied by a disorder of the gastrointestinal tract 3 . Constipation is one of the possible manifestations of dysbacteriosis and microflora disorders associated with taking antibiotics. In this case, as shown by a number of studies, there is a death of normal intestinal microflora, which includes bacteroids, bifidobacteria and lactobacilli, as well as other bacteria useful to us 4 .
With the participation of beneficial bacteria, 3 :
- normally stimulates the immune system;
- final digestion and metabolism of nutrients;
- neutralization and removal of toxic compounds; nine0199 synthesis and supply of B vitamins, pantothenic acid to the body;
- energy supply of the intestinal epithelium, etc.
With the participation of beneficial bacteria, 3 :
- normally stimulates the immune system;
- final digestion and metabolism of nutrients; nine0199 neutralization and removal of toxic compounds;
- synthesis and supply to the body of B vitamins, pantothenic acid;
- energy supply of the intestinal epithelium, etc.
Various components and waste products of bacteria can affect intestinal motility. For example, under the influence of intestinal microflora, short-chain fatty acids are normally formed, which stimulate the motor activity of the intestine. When the microbiome changes, the production of other compounds (such as methane) may increase, which can suppress intestinal motility and lead to unpleasant symptoms 4, 5 .
When the microbiome changes, the production of other compounds (such as methane) may increase, which can suppress intestinal motility and lead to unpleasant symptoms 4, 5 .
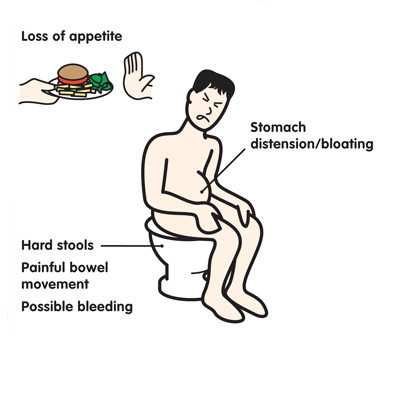
Symptoms of constipation due to medications, including antibiotics, may include 6 :
- abdominal pain, including cramping;
- pain in the rectum and anus;
- loss of appetite;
- bloating; nine0200
- nausea/vomiting;
- infrequent or infrequent, difficult or delayed defecation;
- the need to strain for a long time and often unsuccessfully;
- sensation of incomplete emptying of the bowels after a bowel movement;
- Pain when passing stools due to thickening, roughness, dryness of feces.
 nine0200
nine0200
Symptoms of constipation due to medications, including antibiotics, may include 6 :
- abdominal pain, including cramping;
- pain in the rectum and anus;
- loss of appetite;
- bloating; nine0199 nausea/vomiting;
- infrequent or infrequent, difficult or delayed defecation;
- the need to strain for a long time and often unsuccessfully;
- sensation of incomplete emptying of the bowels after a bowel movement;
- Pain when passing stools due to thickening, roughness, dryness of feces.
 nine0200
nine0200
As a result of inhibition of the normal intestinal microflora, the multiplied opportunistic microbes begin to release toxic substances - toxins. Bacterial toxins have a number of negative effects. They can cause direct damage to the intestinal epithelium, the intestinal mucosa may become thinner and more permeable to pathogens with subsequent development of inflammation 7 . Mucosal damage Intestines can be aggravated by antibiotics themselves, which either have a direct toxic effect on its mucosa, or cause significant changes in the physicochemical properties of intestinal mucus 8 .
How to restore intestinal health after taking antibiotics?
This question worries many patients who have undergone a course of antibiotic therapy. It is better to start treatment of dysbacteriosis as early as possible, as the course of chronic diseases of the digestive tract, if any, can be aggravated. nine0003
It is better to start treatment of dysbacteriosis as early as possible, as the course of chronic diseases of the digestive tract, if any, can be aggravated. nine0003
Therapy should be comprehensive, aimed at eliminating excessive bacterial colonization of the intestine with opportunistic microflora, restoring normal microflora and impaired motility of the large intestine, improving intestinal digestion and absorption, stimulation of the body's reactivity 9 . To normalize the work of the intestine, disturbed during antibiotic therapy, it is recommended to follow a diet. For example, the Mediterranean diet includes an abundance of fruits, vegetables, olive oil and fatty fish (rich in mono- and polyunsaturated fatty acids), whole grains and nuts 10 .
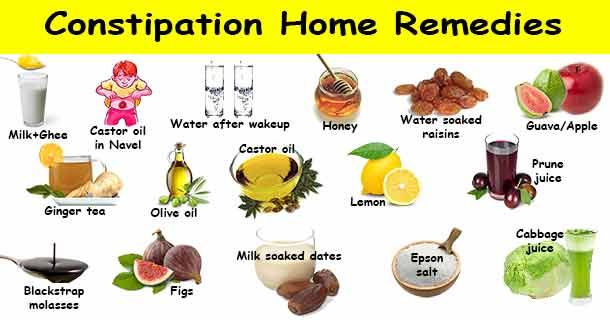
Activation of intestinal motility is facilitated by food rich in dietary fiber. Although dietary fiber is not digested by the gastrointestinal tract's own enzymes, it plays an important role in maintaining growth of beneficial microflora 11 .
The benefits of dietary fiber 11 :
- help to remove harmful substances from the body, including bacterial toxins; nine0200
- retain water, swell and increase the volume of feces, thereby contributing to better bowel movements;
- promote the synthesis of hormones, B vitamins and other substances necessary for the functioning of the immune system;
- serves as food for beneficial gut bacteria. nine0209
- help to remove harmful substances from the body, including bacterial toxins;
- retain water, swell and increase the volume of feces, thereby contributing to better bowel movements;
- promote the synthesis of hormones, B vitamins and other substances necessary for the functioning of the immune system; nine0200
- serves as food for beneficial gut bacteria.

- pomace (this is what remains after squeezing juices from vegetables and fruits, oils from oilseeds) and bran (in fact, waste from the production of high-grade flour), which contain coarse dietary fiber in a high concentration. It is necessary to use them in food in their pure form carefully and in small quantities; nine0200
- whole grains of legumes and cereals and whole grain products thereof;
- nuts and dried fruits - they have less fiber than beans and cereals, but contain a large amount of other biologically active substances necessary for health;
- fresh vegetables, fruits and herbs.
 You need to eat a serving of at least 400 grams per day 12 .
You need to eat a serving of at least 400 grams per day 12 . - use antibiotics only as prescribed by a doctor, but not as self-medication, since they themselves can have a detrimental effect on the normal microflora of the large intestine stimulating the growth of pathogenic and conditionally pathogenic microflora; nine0200
- keep a diet, maintain an optimal drinking regimen;
- reduce the content of simple carbohydrates in food, as they are a good breeding ground for opportunistic bacteria;
- Digestive enzymes may be used to improve digestion.
 nine0209
nine0209
With the participation of beneficial bacteria, 3 normally occurs:
Dietary fiber is found only in plant foods; they are not found in animal products. Many dietary fibers contain 11 :
Fermented milk products play a significant role in the prevention of intestinal dysbacteriosis. They are considered natural probiotics because they contain live microorganisms that have a positive effect on health. with regular use. The menu can include yogurt, kefir, curdled milk, varenets, cottage cheese 3 . To facilitate the excretion of feces, it is necessary to observe the drinking regimen: drink at least 2 liters of fluid per day 11 .
Fermented milk products play a significant role in the prevention of intestinal dysbacteriosis. They are considered natural probiotics because they contain live microorganisms that have a positive effect on health.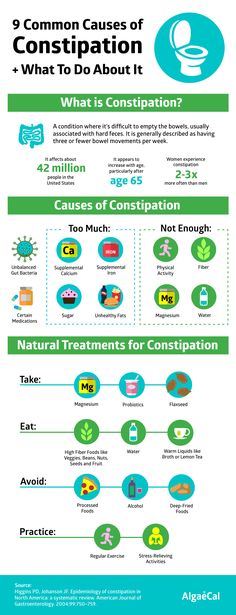 with regular use. The menu can include yogurt, kefir, curdled milk, varenets, cottage cheese 3 . To facilitate the excretion of feces, it is necessary to observe the drinking regimen: drink at least 2 liters of fluid per day 11 .
with regular use. The menu can include yogurt, kefir, curdled milk, varenets, cottage cheese 3 . To facilitate the excretion of feces, it is necessary to observe the drinking regimen: drink at least 2 liters of fluid per day 11 .
Prevention of constipation after antibiotic therapy
To prevent constipation when taking antibiotics, children and adults must adhere to the following rules 9, 13 :
To improve intestinal motility, it is important to be in motion. Moderate physical activity, including abdominal exercises, improves intestinal motility. Hiking, skiing, riding are useful cycling, swimming 11 . When the urge to defecate appears, they cannot be ignored and suppressed. It is important to try to develop a conditioned reflex to empty the bowels at a certain time of the day (the most physiological time of defecation is 15-30 minutes after a hearty breakfast) 11 .
To improve intestinal motility, it is important to be on the move. Moderate physical activity, including abdominal exercises, improves intestinal motility. Hiking, skiing, riding are useful cycling, swimming 11 .
When the urge to defecate appears, they cannot be ignored and suppressed. It is important to try to develop a conditioned reflex to empty the bowels at a certain time of the day (the most physiological time of defecation is 15-30 minutes after a hearty breakfast) 11 .
How to choose a laxative
If constipation due to antibiotics persists despite lifestyle changes, a prescription may be recommended to normalize the condition. laxatives 6 . For the treatment of constipation, drugs of various mechanisms of action are used. Laxatives, e. g. sodium picosulfate (active ingredient of Guttalax®) 14 . Sodium picosulfate is a local laxative, it is activated in the large intestine and has a stimulating effect on the colonic mucosa, promotes the accumulation of water and electrolytes in the intestinal lumen. As a result, the intestinal contents move faster and soften. The laxative effect may occur 6-12 hours after ingestion, therefore, to obtain it in the morning, the medicine should be taken the night before. The drug is available in the form of drops for oral administration and tablets, so there is the possibility of individual dosage selection 14 .
g. sodium picosulfate (active ingredient of Guttalax®) 14 . Sodium picosulfate is a local laxative, it is activated in the large intestine and has a stimulating effect on the colonic mucosa, promotes the accumulation of water and electrolytes in the intestinal lumen. As a result, the intestinal contents move faster and soften. The laxative effect may occur 6-12 hours after ingestion, therefore, to obtain it in the morning, the medicine should be taken the night before. The drug is available in the form of drops for oral administration and tablets, so there is the possibility of individual dosage selection 14 .
More about the drug
If constipation due to antibiotics persists despite lifestyle changes, a prescription may be recommended to normalize the condition. laxatives 6 . For the treatment of constipation, drugs of various mechanisms of action are used. Laxatives, e.g. sodium picosulfate (active ingredient of Guttalax®) 14 . Sodium picosulfate is a local laxative, it is activated in the large intestine and has a stimulating effect on the colonic mucosa, promotes the accumulation of water and electrolytes in the intestinal lumen. As a result, the intestinal contents move faster and soften. The laxative effect may occur 6-12 hours after ingestion, therefore, to obtain it in the morning, the medicine should be taken the night before.
laxatives 6 . For the treatment of constipation, drugs of various mechanisms of action are used. Laxatives, e.g. sodium picosulfate (active ingredient of Guttalax®) 14 . Sodium picosulfate is a local laxative, it is activated in the large intestine and has a stimulating effect on the colonic mucosa, promotes the accumulation of water and electrolytes in the intestinal lumen. As a result, the intestinal contents move faster and soften. The laxative effect may occur 6-12 hours after ingestion, therefore, to obtain it in the morning, the medicine should be taken the night before.