Can zyrtec cause depression
Depression Linked as Side Effect of Some Common Drugs
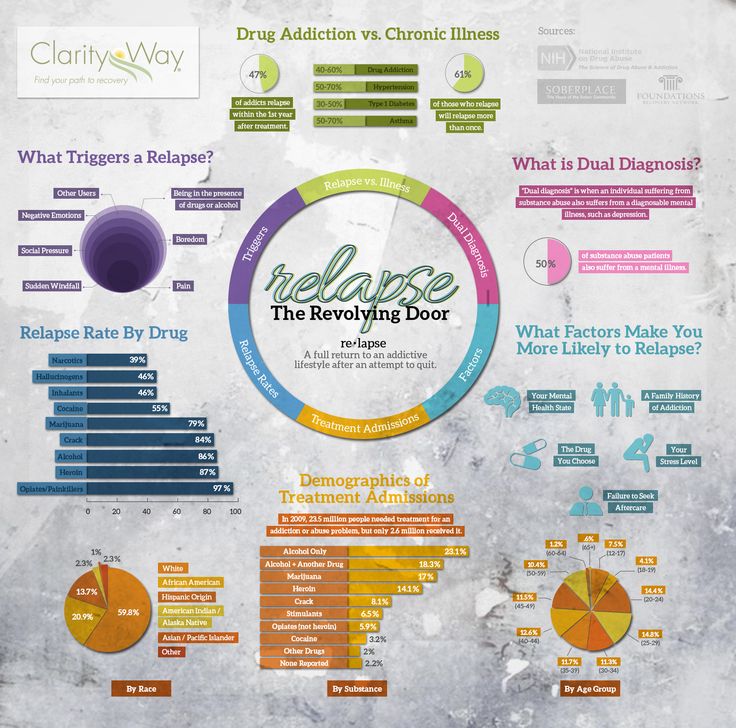
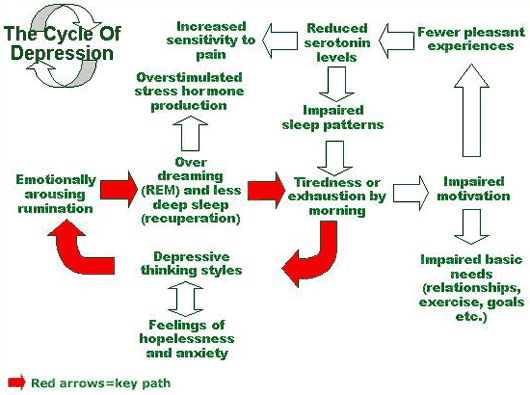
Medications all have some form of side effects, and a recent study revealed one third of Americans are are currently taking medications that may cause the unwanted side effects of depression. Published by the Journal of the American Medical Association the study is the largest of its kind, after previous smaller studies have found similar results.
Approximately 200 medications were found to lead to depression, many of which are prescribed to older adults with ailments like acid reflux and hypertension. What’s concerning is many doctors are unaware that several commonly prescribed medications can increase risk for depression.
The number of drugs taken had a direct impact on risk for depression. While 7 percent of people taking one of the drugs were depressed, those taking three or more resulted in 15. 3 percent with depression. Again this raises concern for older adults who often take more medications, and are more susceptible to their side effects.
“...bear in mind that most people taking these medications, even those who are on three or more of them, don’t have depression...” - Mark Olfson, Columbia University Irving Medical Center
That said, even those taking these drugs associated with depression, may not experience the disorder, and even if they do it may not be caused by the medication. Many individuals have depression before taking the drugs, and others may be experience depression caused by suffering from their various health conditions.
Regardless of the cause of depression, it’s important to seek support if you are experience persistent depression symptoms. Begin by visiting a therapist to assess your symptoms and then provide therapeutic support as needed.
You still will want to be aware of this connection though as changing medication may be the best way to alleviate the depression.
If you believe this study’s results may impact you there are a few things you can do to take steps and avoid or address this potential side effect.
Review Your Medications
If you’re noticing any symptoms of depression you’ll want to talk with your doctor to determine if any of your medications may be the cause. Be sure to consider all of your medications including OTC and prescriptions so you can review them all with your doctor. You should try to this type of medication review once year whether you feel depressed or not. If you feel your doctor is not well-versed on the side effects try to make an appointment to review them with the pharmacist who issues your medication.
Drugs That May Cause Depression
1. Opioids: hydrocodone combination meds (Lorcet, Norco, Vicodin, generic and more) and tramadol (ConZip).
2. Allergy and asthma medications: over-the-counter cetirizine (Zyrtec and generic) and the prescription drug montelukast (Singulair).
3. Over-the-counter proton-pump inhibitors: omeprazole (Prilosec, Zegerid and generic) and esomeprazole (Nexium and generic), as well as the antacids ranitidine (Zantac and generic) and famotidine (Pepcid and generic).
4. Anti-anxiety drugs: alprazolam (Xanax and generic), clonazepam (Klonopin and generic), diazepam (Valium and generic), and lorazepam (Ativan and generic), as well as the sedative zolpidem (Ambien and generic).
5. Antidepressants: sertraline (Zoloft and generic), citalopram (Celexa and generic), bupropion (Wellbutrin and generic) and amitriptyline.
6. Hormones: estradiol (Delestrogen, Elestrin, EstroGel and generic) and finasteride (Proscar, Propecia and generic).
7. Corticosteroids: prednisone and others.
8. Anticonvulsants: gabapentin (Neurontin and generic) and topiramate (Topamax and generic).
9. Beta blockers and angiotensin-converting-enzyme inhibitors (blood pressure drugs): metoprolol, atenolol, enalapril and quinapril.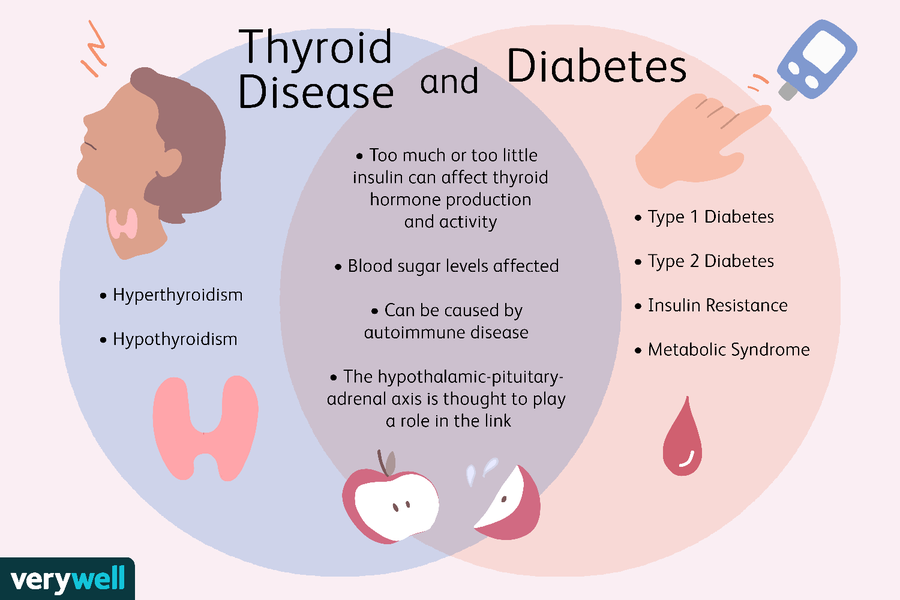
Be Aware of Your Mood
Whether you take any of these medications or not, be sure to discuss your mood if you’re feeling low for two or more consecutive weeks. Other symptoms may include appetite or weight changes, loss of enjoyment in activities, severe fatigue, sleep issues, inability to concentrate, feeling hopeless or worthless, or recurrent thoughts of suicide or death.
Monitor New Meds
Depression as a side effect may appear at any time, but it’s most common to notice it within the first month of use. If you doctor prescribes a new medication, first ask if depression is a side effect. If it is, start to note any mood related side effects you may notice and include the time and day it occurs. If any symptoms last more than a week or two, report them to a doctor immediately.
Change Medication
If you believe medication is causing depression, discuss it with your doctor. One solution may be altering the dose. If the lower dose helps you mood but no longer address the primary need for the medicine your doctor may need to change your prescription to another class of drug. Depending on your health, you may be able to terminate the use of the drug altogether, but be certain to discuss this with your doctor to determine if that’s possible and guide you on how to wean off of the medication properly.
Depending on your health, you may be able to terminate the use of the drug altogether, but be certain to discuss this with your doctor to determine if that’s possible and guide you on how to wean off of the medication properly.
Lifestyle Changes
If your ailments do not allow for changing or stopping medication, you should discuss with your doctor lifestyle changes you can make to boost your mood. Incorporate a simple exercise routine, may be an easy way to help improve your mood. You may also consider talk therapy, or trying an antidepressant- just be mindful same may increase anxiety or worsen depression.
Assessment of the effects of antihistamine drugs on mood, sleep quality, sleepiness, and dream anxiety
Randomized Controlled Trial
. 2014 Aug;18(3):161-8.
doi: 10.3109/13651501.2014.907919. Epub 2014 Apr 23.
Pinar Guzel Ozdemir 1 , Ayşe Serap Karadag, Yavuz Selvi, Murat Boysan, Serap Gunes Bilgili, Adem Aydin, Sevda Onder
Affiliations
Affiliation
- 1 Department of Psychiatry, Yuzuncu Yil University Medicine Faculty , Van , Turkey.
- PMID: 24673474
- DOI: 10.3109/13651501.2014.907919
Randomized Controlled Trial
Pinar Guzel Ozdemir et al. Int J Psychiatry Clin Pract. 2014 Aug.
. 2014 Aug;18(3):161-8.
doi: 10. 3109/13651501.2014.907919. Epub 2014 Apr 23.
3109/13651501.2014.907919. Epub 2014 Apr 23.
Authors
Pinar Guzel Ozdemir 1 , Ayşe Serap Karadag, Yavuz Selvi, Murat Boysan, Serap Gunes Bilgili, Adem Aydin, Sevda Onder
Affiliation
- 1 Department of Psychiatry, Yuzuncu Yil University Medicine Faculty , Van , Turkey.
- PMID: 24673474
- DOI: 10.3109/13651501.2014.907919
Abstract
Objective: There are limited comparative studies on classic and new-generation antihistamines that affect sleep quality and mood. The purpose of this study was to determine and compare the effects of classic and new-generation antihistamines on sleep quality, daytime sleepiness, dream anxiety, and mood.
The purpose of this study was to determine and compare the effects of classic and new-generation antihistamines on sleep quality, daytime sleepiness, dream anxiety, and mood.
Methods: Ninety-two patients with chronic pruritus completed study in the dermatology outpatient clinic. Treatments with regular recommended therapeutic doses were administered. The effects of antihistaminic drugs on mood, daytime sleepiness, dream anxiety, and sleep quality were assessed on the first day and 1 month after.
Results: Outpatients who received cetirizine and hydroxyzine treatments reported higher scores on the depression, anxiety, and fatigue sub-scales than those who received desloratadine, levocetirizine, and rupatadine. Pheniramine and rupatadine were found to be associated with daytime sleepiness and better sleep quality. UKU side effects scale scores were significantly elevated among outpatients receiving pheniramine. Classic antihistamines increased daytime sleepiness and decreased the sleep quality scores. New-generation antihistamines reduced sleep latency and dream anxiety, and increased daytime sleepiness and sleep quality.
Classic antihistamines increased daytime sleepiness and decreased the sleep quality scores. New-generation antihistamines reduced sleep latency and dream anxiety, and increased daytime sleepiness and sleep quality.
Conclusion: Both antihistamines, significantly increased daytime sleepiness and nocturnal sleep quality. Daytime sleepiness was significantly predicted by rupadatine and pheniramine treatment. Cetirizine and hydroxyzine, seem to have negative influences on mood states. Given the extensive use of antihistamines in clinical settings, these results should be more elaborately examined in further studies.
Keywords: Antihistamines; daytime sleepiness; dream anxiety; mood; sleep quality.
Similar articles
-
Night-time sedating h2 -antihistamine increases daytime somnolence but not treatment efficacy in chronic spontaneous urticaria: a randomized controlled trial.

Staevska M, Gugutkova M, Lazarova C, Kralimarkova T, Dimitrov V, Zuberbier T, Church MK, Popov TA. Staevska M, et al. Br J Dermatol. 2014 Jul;171(1):148-54. doi: 10.1111/bjd.12846. Epub 2014 May 26. Br J Dermatol. 2014. PMID: 24472058 Free PMC article. Clinical Trial.
-
Brain histamine h2 receptor occupancy measured by PET after oral administration of levocetirizine, a non-sedating antihistamine.
Hiraoka K, Tashiro M, Grobosch T, Maurer M, Oda K, Toyohara J, Ishii K, Ishiwata K, Yanai K. Hiraoka K, et al. Expert Opin Drug Saf. 2015 Feb;14(2):199-206. doi: 10.1517/14740338.2015.989831. Epub 2014 Dec 3. Expert Opin Drug Saf. 2015. PMID: 25466429 Clinical Trial.
-
Central effects of fexofenadine and cetirizine: measurement of psychomotor performance, subjective sleepiness, and brain histamine h2-receptor occupancy using 11C-doxepin positron emission tomography.
Tashiro M, Sakurada Y, Iwabuchi K, Mochizuki H, Kato M, Aoki M, Funaki Y, Itoh M, Iwata R, Wong DF, Yanai K. Tashiro M, et al. J Clin Pharmacol. 2004 Aug;44(8):890-900. doi: 10.1177/0091270004267590. J Clin Pharmacol. 2004. PMID: 15286093 Clinical Trial.
-
Second-generation antihistamines for the treatment of chronic idiopathic urticaria.
Belsito DV. Belsito DV. J Drugs Dermatol. 2010 May;9(5):503-12. J Drugs Dermatol. 2010. PMID: 20480793 Review.
-
Antihistamines in dermatology.
Greaves MW. Greaves MW. Skin Pharmacol Physiol. 2005 Sep-Oct;18(5):220-9. doi: 10.1159/000086667. Epub 2005 Jul 5. Skin Pharmacol Physiol. 2005. PMID: 16015020 Review.
See all similar articles
Cited by
-
Histamine and histamine receptors: Roles in major depressive disorder.
Qian H, Shu C, Xiao L, Wang G. Qian H, et al. Front Psychiatry. 2022 Sep 23;13:825591. doi: 10.3389/fpsyt.2022.825591. eCollection 2022. Front Psychiatry. 2022. PMID: 36213905 Free PMC article. Review.
-
Impaired sleep quality in children with allergic conjunctivitis and their parents.
Li J, Zhang SY, Fan Z, Liu R, Jin L, Liang L. Li J, et al. Eye (Lond). 2022 Jul 22. doi: 10.1038/s41433-022-02182-4. Online ahead of print. Eye (Lond). 2022. PMID: 35869391
-
Ameliorative Effect of a Neoteric Regimen of Catechin plus Cetirizine on Ovalbumin-Induced Allergic Rhinitis in Rats.
Morsy MA, Patel SS, Bakrania A, Kandeel M, Nair AB, Shah JN, Akrawi SH, El-Daly M. Morsy MA, et al. Life (Basel).
 2022 May 31;12(6):820. doi: 10.3390/life12060820. Life (Basel). 2022. PMID: 35743851 Free PMC article.
2022 May 31;12(6):820. doi: 10.3390/life12060820. Life (Basel). 2022. PMID: 35743851 Free PMC article. -
Frequently reported adverse events of rebamipide compared to other drugs for peptic ulcer and gastroesophageal reflux disease.
Jang E, Park M, Jeong JE, Lee JY, Kim MG. Jang E, et al. Sci Rep. 2022 May 12;12(1):7839. doi: 10.1038/s41598-022-11505-0. Sci Rep. 2022. PMID: 35552457 Free PMC article.
-
Pathobiology of Second-Generation Antihistamines Related to Sleep in Urticaria Patients.
Mann C, Wegner J, Weeß HG, Staubach P. Mann C, et al. Biology (Basel). 2022 Mar 11;11(3):433. doi: 10.3390/biology11030433. Biology (Basel). 2022. PMID: 35336805 Free PMC article.
See all "Cited by" articles
Publication types
MeSH terms
Substances
Instructions for use, home delivery
Characteristics
| Pack quantity | 1 piece |
| Minimum allowable storage temperature, °С | 25 °C |
| Maximum allowable storage temperature, °С | nine0007 10 °C|
| Expiration date | 3 months |
| Form | Cream |
| Active ingredient | Cetirizine dihydrochloride |
| Pharmacological group | Histamine H1 receptor blocker. Antiallergic drug Antiallergic drug |
Description
Anti -allergic agent
Active substances
Cetirizis
Form of output
drops
Composition
0052
Cetirizine dihydrochloride 10 mg.
Excipients: methyl parahydroxybenzoate, propyl parahydroxybenzoate, glycerol, propylene glycol, sodium saccharinate dihydrate, sodium acetate trihydrate, glacial acetic acid, purified water.
Pharmacological effect
Cetirizine is a hydroxyzine metabolite, belongs to the group of competitive histamine antagonists, blocks histamine H1 receptors.
In addition to the antihistamine effect, cetirizine prevents the development and alleviates the course of allergic reactions: at a dose of 10 mg 1 or 2 times / day, it inhibits the late phase of eosinophil aggregation in the skin and conjunctiva of atopic patients. After oral administration, the antiallergic effect of cetirizine lasts for 24 hours.
Clinical efficacy and safety
Studies in healthy volunteers have shown that cetirizine at doses of 5 or 10 mg significantly inhibits the rash and redness response to high concentrations of histamine in the skin, but no correlation with efficacy has been established.
In a 6-week, placebo-controlled study of 186 patients with allergic rhinitis and concomitant mild to moderate asthma, cetirizine 10 mg once daily was shown to reduce symptoms of rhinitis and not affect lung function. nine0003
The results of this study confirm the safety of the use of cetirizine in patients with allergies and mild to moderate bronchial asthma.
In a placebo-controlled study, cetirizine 60 mg daily for 7 days did not cause clinically significant QT interval prolongation.
The use of cetirizine at the recommended dose has been shown to improve the quality of life of patients with year-round and seasonal allergic rhinitis. nine0003
Children
In a 35-day study in patients aged 5-12 years, there was no evidence of resistance to the antihistamine effect of cetirizine. The normal skin reaction to histamine was restored within 3 days after discontinuation of the drug with repeated use.
The normal skin reaction to histamine was restored within 3 days after discontinuation of the drug with repeated use.
A 7-day, placebo-controlled study of cetirizine syrup in 42 patients aged 6 to 11 months demonstrated safety of the drug. Cetirizine was administered at a dose of 0.25 mg/kg 2 times a day, which corresponded approximately to 4.5 mg per day (dose range was from 3.4 to 6.2 mg per day). nine0003
Use in children aged 6 to 12 months is possible only on prescription and under strict medical supervision.
Pharmacokinetics
Pharmacokinetic parameters of cetirizine when used in doses of 5 to 60 mg change linearly.
Absorption
Cmax in blood plasma is achieved after 1±0.5 h and is 300 ng/ml. Various pharmacokinetic parameters such as Cmax and AUC are homogeneous. Eating does not affect the complete absorption of cetirizine, although its rate decreases. nine0003
The bioavailability of various dosage forms of cetirizine (solution, capsules, tablets) is comparable.
Distribution
Plasma protein binding is approximately 93±0.3%. Vd is 0.5 l/kg. Cetirizine does not affect the binding of warfarin to proteins.
When administered orally at a daily dose of 10 mg for 10 days, no accumulation of cetirizine was observed.
Metabolism
Cetirizine does not undergo extensive primary metabolism. nine0003
Elimination
T1 / 2 is approximately 10 hours. Approximately 2/3 of the dose taken is excreted in the urine unchanged.
Pharmacokinetics in special clinical situations
Elderly patients. In 16 elderly patients, with a single dose of the drug at a dose of 10 mg, T1 / 2 was 50% higher, and the clearance was 40% lower compared to younger patients. Decreased clearance of cetirizine in elderly patients is likely due to a decrease in renal function in this category of patients. nine0003
Patients with renal insufficiency. In patients with mild renal insufficiency (CC> 40 ml / min), pharmacokinetic parameters are similar to those in healthy volunteers with normal renal function. In patients with moderate renal insufficiency and in patients on hemodialysis (QC
In patients with moderate renal insufficiency and in patients on hemodialysis (QC
Patients with hepatic insufficiency. In patients with chronic liver diseases (hepatocellular, cholestatic and biliary cirrhosis), with a single dose of the drug at a dose of 10 or 20 mg, T1 / 2 increases by about 50%, and clearance decreases by 40% compared with healthy volunteers. Dose adjustment is necessary only if a patient with hepatic impairment also has concomitant renal impairment. nine0003
Children. In children aged 6 to 12 years, T1 / 2 is 6 hours, at the age of 2 to 6 years - 5 hours, at the age of 6 months to 2 years it is reduced to 3.1 hours. symptoms of year-round (persistent) and seasonal (intermittent) allergic rhinitis and allergic conjunctivitis (itching, sneezing, nasal congestion, rhinorrhea, lacrimation, conjunctival hyperemia).
Symptoms of chronic idiopathic urticaria.
Contraindications
Hypersensitivity to cetirizine, hydroxyzine or piperazine derivatives, as well as to other components of the drug.
End stage renal disease (CC
Children under 6 months of age (due to limited efficacy and safety data).
Pregnancy.
Precautions:
Chronic renal insufficiency (CC>10 ml / min, dosing regimen adjustment required). nine0003
Epilepsy and increased convulsive readiness.
Patients with predisposing factors for urinary retention (spinal cord injury, prostatic hyperplasia).
Elderly patients (with age-related decrease in GFR).
Children under 1 year of age.
Breastfeeding period.
Pregnancy and lactation
Pregnancy
Prospective analysis of more than 700 pregnancy outcomes revealed no malformations, fetal or neonatal toxicity with a clear causal relationship. nine0003
Animal studies have not revealed any direct or indirect adverse effects of cetirizine on the developing fetus (including in the postnatal period), pregnancy and postnatal development. Adequate and strictly controlled clinical studies on the safety of the drug during pregnancy have not been conducted, so Zodak should not be used during pregnancy.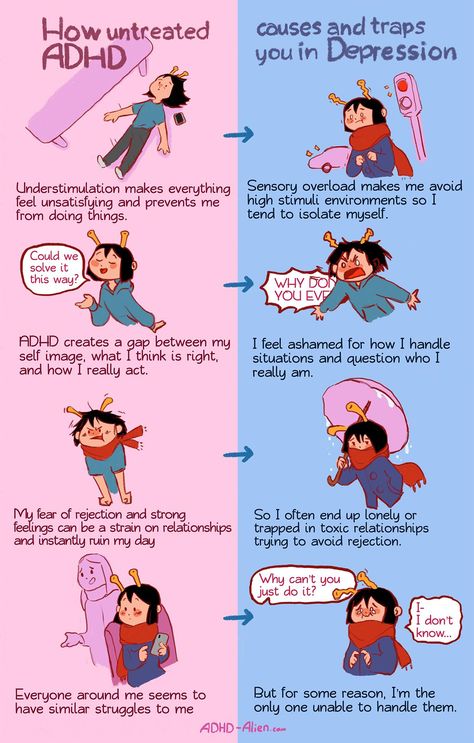
Breastfeeding
Cetirizine is excreted in breast milk in an amount of 25% to 90% concentration of the drug in plasma, depending on the time after administration. During breastfeeding, use after consulting a doctor if the intended benefit to the mother outweighs the potential risk to the child.
Fertility
Available data on the effects on human fertility are limited, but no adverse effects on fertility have been identified.
Dosage and administration
Inside, drip into a spoon or dissolve in water. The amount of water to dissolve the drug should correspond to the amount of liquid that the patient (especially the child) is able to swallow. The solution should be taken immediately after preparation. nine0003
Adults 10 mg (20 drops) once daily.
Elderly patients. There is no need to reduce the dose in elderly patients if renal function is not impaired.
Patients with renal insufficiency. Since cetirizine is excreted from the body mainly by the kidneys, if alternative treatment is not possible in patients with renal insufficiency, the dosage regimen of the drug should be adjusted depending on renal function (CC value).
Side effects
From the side of the hematopoietic system: very rarely - thrombocytopenia.
From the immune system: rarely - hypersensitivity reactions, very rarely - anaphylactic shock.
Mental disorders: infrequently - agitation, rarely - aggression, confusion, depression, hallucinations, sleep disturbance, very rarely - tic, frequency unknown - suicidal ideation.
From the nervous system: infrequently - paresthesia, rarely - convulsions, very rarely - taste perversion, dyskinesia, dystonia, fainting, tremor, frequency unknown - memory impairment, incl. amnesia. nine0003
On the part of the organ of vision: very rarely - disturbance of accommodation, blurred vision, nystagmus.
From the side of the organ of hearing: the frequency is unknown - vertigo, deafness.
From the side of the cardiovascular system: rarely - tachycardia, the frequency is unknown - vasculitis.
From the digestive system: infrequently - diarrhea, rarely - changes in liver function tests (increased activity of transaminases, alkaline phosphatase, GGT and bilirubin), the frequency is unknown - increased appetite.
From the skin and subcutaneous tissues: infrequently - skin rash, pruritus, rarely - urticaria, very rarely - angioedema, persistent drug erythema.
From the urinary system: very rarely - dysuria, enuresis, the frequency is unknown - urinary retention.
From the musculoskeletal system: the frequency is unknown - arthralgia.
General disorders: infrequently - asthenia, malaise, rarely - peripheral edema, weight gain.
Description of selected adverse reactions
Cases of pruritus (including intense pruritus) and/or urticaria have been reported following discontinuation of cetirizine.
Overdose
The clinical picture observed in overdose of cetirizine was due to its effect on the central nervous system.
Symptoms: after a single dose of cetirizine at a dose of 50 mg, the following clinical picture was observed - confusion, dizziness, drowsiness, lethargy, stupor, weakness, anxiety, irritability, sedation, fatigue, malaise, headache, mydriasis, itching, tachycardia, tremor, urinary retention, dry mouth, diarrhea, constipation. nine0003
nine0003
Treatment: gastric lavage or induction of vomiting should be carried out immediately after taking the drug. The appointment of activated charcoal, symptomatic and supportive therapy is recommended. The specific antidote is unknown. Hemodialysis is ineffective.
Interaction with other drugs
No clinically significant interaction of cetirizine with other drugs has been established.
Based on the results of drug interaction studies with cetirizine, in particular, interaction studies with pseudoephedrine or theophylline at a dose of 400 mg / day, no clinically significant interaction has been established. nine0003
Simultaneous use of cetirizine with ethanol and drugs that depress the central nervous system may further reduce concentration and reaction time, although cetirizine does not enhance the effect of ethanol (at its blood concentration of 0.5 g / l).
Special instructions
cetirizine may increase the risk of urinary retention. nine0003
nine0003
Caution is advised when using cetirizine concomitantly with alcohol, although no clinically significant interaction with ethanol has been observed at therapeutic doses (at a blood ethanol concentration of 0.5 g/l).
Caution should be exercised in patients with epilepsy and increased convulsive readiness.
A three-day "wash-out" period is recommended before allergological testing because histamine H1 receptor blockers inhibit the development of allergic skin reactions. nine0003
Pediatric use
Due to potential CNS depressant effects, caution should be exercised when prescribing Zodak to children under 1 year of age with the following risk factors for sudden infant death syndrome, such as (but not limited to):
sibling sleep apnea or sudden infant death syndrome,
maternal drug or smoking abuse during pregnancy,
young age of the mother (19 years and younger),
excessive smoking of the babysitter (1 pack of cigarettes per day or more),
children who regularly fall asleep face down and who are not laid on their backs,
preterm ( less than 37 weeks' gestational age) or underweight (below 10th percentile of gestational age) infants,
when co-administered with CNS depressant drugs.
The preparation contains excipients methylparabenzene and propylparabenzene, which can cause allergic reactions, incl. slow type. nine0003
Influence on the ability to drive vehicles and mechanisms
An objective assessment of the ability to drive vehicles and work with mechanisms did not reliably reveal any adverse events when using Zodak in recommended doses. However, for patients with manifestations of drowsiness while taking the drug during the period of treatment, it is advisable to refrain from driving, engaging in potentially hazardous activities or operating mechanisms that require increased concentration and speed of psychomotor reactions. nine0003
Storage conditions
Store under normal conditions, out of the reach of children.
Zyrtec drops orally 10mg/ml 20ml with free home delivery from VkusVill
VkusVill
Transparent colorless liquid with the smell of acetic acid.
Vkusvill
205 rub/piece 205. 00 205.00
00 205.00
from the partner "Apteka DIALOG Stolitsa"
Description
Transparent colorless liquid with the smell of acetic acid.
Indications
Cetirizine dihydrochloride, oral drops 10 mg/ml, is indicated in adults and children 6 months of age and older for the relief of: - nasal and ocular symptoms of year-round (persistent) and seasonal (intermittent) allergic rhinitis and allergic conjunctivitis: itching, sneezing, nasal congestion, rhinorrhea, lacrimation, conjunctival hyperemia; - symptoms of chronic idiopathic urticaria. Use in children from 6 to 12 months is possible only on prescription and under strict medical supervision. nine0003 Producer
YUSB Farshim/Aysika Pharmaceuticals
INN/Active ingredient Dosage10 mg/ml
Packing form Quantity per pack Ingredients Method of preparation Special conditions Side effects Contraindications Storage conditions Expiry date Minimum age Prescription drug PartnerPharmacy DIALOG Capital
This product can be supplied by several manufacturers at once.