Can you take prozac while breastfeeding
Pregnancy, breastfeeding and fertility while taking fluoxetine
Fluoxetine and pregnancy
You can take fluoxetine during pregnancy.
Some studies have suggested that fluoxetine might occasionally affect the development of a baby’s heart. However, if there is any risk, it is small, and most babies born to women taking fluoxetine have a normal heart.
When fluoxetine is taken in the weeks before delivery it can sometimes cause short-term withdrawal symptoms and, very rarely, breathing problems in the baby. Your baby will be checked after birth and given extra care if needed.
Taking fluoxetine in the last month of pregnancy may slightly increase your risk of bleeding after delivery. However, because this side effect is rare, it's not a reason to stop taking fluoxetine.
It's important that mental health problems are well treated since these can affect your wellbeing and your baby’s. Depression and anxiety can sometimes get worse during pregnancy, and after a baby’s born.
Speak to your doctor if you become pregnant while taking fluoxetine. They will help you weigh up the risks and benefits so you can decide on the best treatment for you and your baby.
Fluoxetine and breastfeeding
If your doctor or health visitor says your baby is healthy, you can take fluoxetine while breastfeeding.
Fluoxetine passes into breast milk, usually in fairly small amounts. It has been linked with side effects in a few breastfed babies, but has been used by many breastfeeding mothers without any problems.
Although other medicines that pass into breast milk in smaller amounts might be preferred, it's important you take the medicine that works for you if you are breastfeeding, or planning to breastfeed. Talk to your doctor or pharmacist to help you decide.
It's important to keep taking fluoxetine to keep you well. Breastfeeding will also benefit both you and your baby.
If you notice that your baby is not feeding as well as usual, seems unusually sleepy or irritable, has colic, or if you have any other concerns about your baby, then talk to your health visitor, midwife, pharmacist or doctor as soon as possible.
Fluoxetine and fertility
There is no evidence to suggest that taking fluoxetine reduces fertility in either men or women. But speak to a pharmacist or your doctor if you're trying to get pregnant. They may want to review your treatment.
Non-urgent advice: Tell your doctor if you're:
- trying to get pregnant
- pregnant
- breastfeeding
Find out about how fluoxetine can affect you and your baby during pregnancy on the Best Use of Medicines in Pregnancy (BUMPS) website.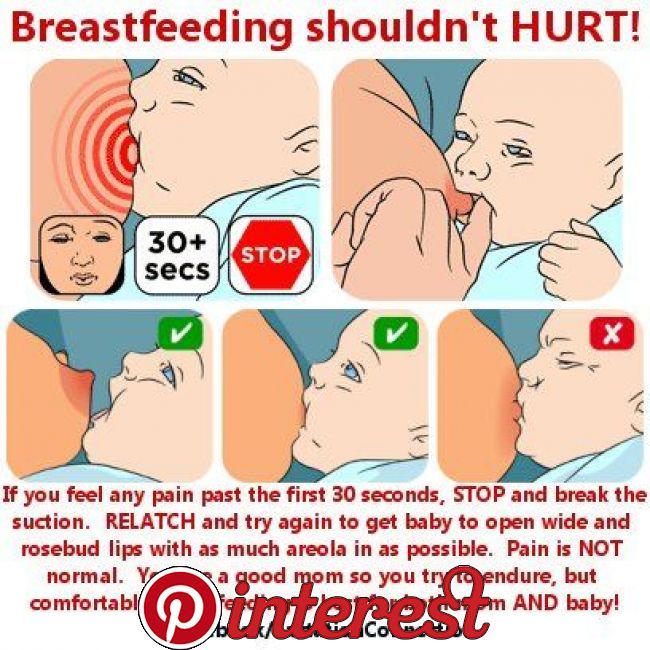
Page last reviewed: 10 February 2022
Next review due: 10 February 2025
Fluoxetine - Drugs and Lactation Database (LactMed®)
Summary of Use during Lactation
The average amount of drug in breastmilk is higher with fluoxetine than with most other SSRIs and the long-acting, active metabolite, norfluoxetine, is detectable in the serum of most breastfed infants during the first 2 months postpartum and in a few thereafter. Adverse effects such as colic, fussiness, and drowsiness have been reported in some breastfed infants. Decreased infant weight gain was found in one study, but not in others. No adverse effects on development have been found in a few infants followed for up to a year.
If fluoxetine is required by the mother, it is not a reason to discontinue breastfeeding. A safety scoring system finds fluoxetine use to be possible during breastfeeding,[1] although others do not recommend its use. [2] If the mother was taking fluoxetine during pregnancy or if other antidepressants have been ineffective, most experts recommend against changing medications during breastfeeding. Otherwise, agents with lower excretion into breastmilk may be preferred, especially while nursing a newborn or preterm infant. The breastfed infant should be monitored for behavioral side effects such as colic, agitation, irritability, poor feeding, and poor weight gain.
[2] If the mother was taking fluoxetine during pregnancy or if other antidepressants have been ineffective, most experts recommend against changing medications during breastfeeding. Otherwise, agents with lower excretion into breastmilk may be preferred, especially while nursing a newborn or preterm infant. The breastfed infant should be monitored for behavioral side effects such as colic, agitation, irritability, poor feeding, and poor weight gain.
Mothers taking an SSRI during pregnancy and postpartum may have more difficulty breastfeeding, although this might be a reflection of their disease state.[3] These mothers may need additional breastfeeding support. Breastfed infants exposed to an SSRI during the third trimester of pregnancy have a lower risk of poor neonatal adaptation than formula-fed infants.
Drug Levels
Fluoxetine is metabolized to norfluoxetine which has antidepressant activity that is considered to be equal to fluoxetine.[2]
Maternal Levels. In a pooled analysis of serum levels from published studies and 1 unpublished case, the authors found that 20 mothers taking an average daily dosage of 28 mg (range 10 to 80 mg) had an average milk fluoxetine level of 76 mcg/L (range 23 to 189 mcg/L).[4] Using the average dosage and milk level data from this paper, an exclusively breastfed infant would receive an estimated 2.4% of the maternal weight-adjusted dosage of fluoxetine; however, the substantial contribution of norfluoxetine was not considered. In one of the studies included in the pooled analysis that measured both fluoxetine and norfluoxetine in 14 mothers, the average daily infant dosage in breastmilk was about 7% (range 3 to 12%) of the mother's weight-adjusted dosage.[4]
In a pooled analysis of serum levels from published studies and 1 unpublished case, the authors found that 20 mothers taking an average daily dosage of 28 mg (range 10 to 80 mg) had an average milk fluoxetine level of 76 mcg/L (range 23 to 189 mcg/L).[4] Using the average dosage and milk level data from this paper, an exclusively breastfed infant would receive an estimated 2.4% of the maternal weight-adjusted dosage of fluoxetine; however, the substantial contribution of norfluoxetine was not considered. In one of the studies included in the pooled analysis that measured both fluoxetine and norfluoxetine in 14 mothers, the average daily infant dosage in breastmilk was about 7% (range 3 to 12%) of the mother's weight-adjusted dosage.[4]
Eleven women taking fluoxetine 20 to 40 mg daily during pregnancy and lactation had trough milk fluoxetine and norfluoxetine levels measured on day 4, week 2 and month 2 postpartum. When standardized to a 20 mg daily dosage, total drug concentration in breastmilk ranged from 75. 4 to 91.5 mcg/L at the 3 times. The authors estimated that an exclusively breastfed infant would receive a minimum of 2.4% and 3.8% of the maternal weight-adjusted dosage of the drug and metabolite combined with this maternal dosage regimen at 2 weeks and 2 months of age, respectively.[5]
4 to 91.5 mcg/L at the 3 times. The authors estimated that an exclusively breastfed infant would receive a minimum of 2.4% and 3.8% of the maternal weight-adjusted dosage of the drug and metabolite combined with this maternal dosage regimen at 2 weeks and 2 months of age, respectively.[5]
In 1 mother who was 11 weeks postpartum and taking fluoxetine in a daily dosage of 20 mg, the authors estimated that an exclusively breastfed infant would receive 3.3% of the maternal weight-adjusted dosage.[6]
At 2 months postpartum, 7 mothers taking an average of 24 mg of fluoxetine daily had average breastmilk levels of 63.7 mcg/L of fluoxetine and 103.8 mcg/L of norfluoxetine. These data indicate that a fully breastfed infant would receive 25 mcg/kg daily of fluoxetine plus norfluoxetine. This value would be about 6.3% of the maternal weight-adjusted dosage.[7]
Twenty-three nursing mothers who were taking fluoxetine in an average dose of 21 mg daily for a minimum of 4 weeks and averaging 3. 7 months postpartum had random foremilk samples (n = 30) analyzed for R- and S-isomers of fluoxetine and norfluoxetine. The weight-adjusted dosages of these infants (average 91% breastfed) were calculated to be 0.54% and 0.57% of the maternal weight-adjusted dosage for fluoxetine and norfluoxetine, respectively. The concentrations of the active S-isomer in milk were about 1.9 times that of the R- isomer.[8]
7 months postpartum had random foremilk samples (n = 30) analyzed for R- and S-isomers of fluoxetine and norfluoxetine. The weight-adjusted dosages of these infants (average 91% breastfed) were calculated to be 0.54% and 0.57% of the maternal weight-adjusted dosage for fluoxetine and norfluoxetine, respectively. The concentrations of the active S-isomer in milk were about 1.9 times that of the R- isomer.[8]
Data from 24 women on the excretion of fluoxetine and norfluoxetine into milk from two previously published studies[9,10] were combined and reanalyzed using NONMEM. Data from the two original papers were previously included in a pooled analysis reported above.[4] Simulations of the data indicated that a fully breastfed infant would receive a median of 0.017 mg/kg daily of fluoxetine plus norfluoxetine. This resulted in a median weight-adjusted percentage of 5.9% of the maternal dosage, with a 99th percentile value of 23%.[11]
Four women taking fluoxetine 20 mg daily had an average breastmilk fluoxetine concentration of 51 mcg/L at times that were not stated. These values equated to an average infant dosage of 7.6 mcg/kg daily or 2.2% of the maternal weight-adjusted dosage.[12]
These values equated to an average infant dosage of 7.6 mcg/kg daily or 2.2% of the maternal weight-adjusted dosage.[12]
A nursing mother was taking fluoxetine 20 mg daily. Foremilk and hindmilk samples taken at 1 week postpartum, 2 hours after a dose contained 19 mcg/L and 16 mcg/L, respectively. The samples also contained 36 mcg/L and 30 mcg/L of norfluoxetine in foremilk and hindmilk, respectively.[13]
A mother taking 40 mg of fluoxetine daily since conception donated one mature breastmilk sample 4 hours after a dose. Fluoxetine concentration was 98.2 mcg/L and norfluoxetine was 56.3 mcg/L. In 17 women who received a single 20 mg dose of fluoxetine prepartum, colostrum samples taken 23 to 54 hours postpartum. Fluoxetine was found in 3 samples below the quantification level of 3 mcg/L and above the level of detection of 1 mcg/L; in all other samples fluoxetine was below the level of detection. Norfluoxetine was found in all the samples, but below the level of quantification of 4 mcg/L. [14]
[14]
Two women treated with fluoxetine 20 mg daily during the third trimester of pregnancy and during breastfeeding provided trough milk samples during the first week postpartum. Fluoxetine milk levels were 142.9 and 216.8 mcg/L, with calculated weight-adjusted percentage of maternal dosages 13.1% and 20%, respectively. These values might be inaccurate because of the timing of milk sample collection and the failure to quantitate norfluoxetine.[15]
Infant Levels. In a pooled analysis of 22 mother-infant pairs from published and unpublished cases, the authors found that infants had an average of 7% (range 0 to 59%) of their mothers' fluoxetine plasma levels; 4 of the 22 infants (18%) had a plasma level greater than 10% of the mothers' which was defined by the authors as being elevated.[4]
An infant whose mother restarted fluoxetine at 63 days postpartum had blood drawn after 2 days of breastfeeding. The infant’s fluoxetine level was 340 mcg/L and norfluoxetine was 208 mcg/L. [16]
[16]
A mother took fluoxetine 40 mg daily during pregnancy and postpartum. Infant blood samples were taken at two times. The first was at approximately 11 days of age. The fluoxetine concentration was less than 40 mcg/L and norfluoxetine was 142 mcg/L. Eight days later after breastfeeding was discontinued, the infant’s fluoxetine concentration was less than 40 mcg/L and norfluoxetine was 86 mcg/L.[17]
Eleven breastfed (6 exclusively; 5 supplemented) infants with an average age of 24.6 weeks (range 5 to 36 weeks) whose mothers were taking an average of 27.3 mg daily of fluoxetine all had measurable norfluoxetine serum levels averaging 3.2 mcg/L (range 1.4 to 8.7 mcg/L) which was 3.2% of average maternal norfluoxetine serum levels. One infant also had a detectable fluoxetine level of 2.6 mcg/L.[18]
The breastfed (extent not stated) infants of 11 women taking fluoxetine 20 to 40 mg daily during pregnancy and lactation had trough milk fluoxetine and norfluoxetine levels measured on day 4, week 2 and month 2 postpartum. At 2 weeks of age, fluoxetine was detectable in the serum of 2 of the infants in concentrations of 7.1% of the average maternal serum level. Norfluoxetine was detectable in the serum of all infants in average concentrations of 38% of the average maternal serum level. At 2 months of age, no infant had detectable fluoxetine levels; norfluoxetine was detectable in the serum of all infants in concentrations averaging 6.5% of the average maternal serum level.[5]
At 2 weeks of age, fluoxetine was detectable in the serum of 2 of the infants in concentrations of 7.1% of the average maternal serum level. Norfluoxetine was detectable in the serum of all infants in average concentrations of 38% of the average maternal serum level. At 2 months of age, no infant had detectable fluoxetine levels; norfluoxetine was detectable in the serum of all infants in concentrations averaging 6.5% of the average maternal serum level.[5]
At 2 months postpartum, the breastfed infants of 7 mothers taking an average of 24 mg of fluoxetine daily had an average serum levels of 2 mcg/L of fluoxetine and 8.5 mcg/L of norfluoxetine which was about 9% of the maternal serum norfluoxetine level.[7]
Thirty serum levels were obtained from 23 infants with an average age of 3.7 months and breastfed an average of 91% by mothers who had been taking fluoxetine in an average dose of 21 mg daily for at least 4 weeks. Six of 7 infants had detectable serum levels in the first month, 6 of 8 in the second month and 2 of 14 thereafter. The ratio of infant to maternal serum levels of fluoxetine plus S-norfluoxetine dropped rapidly during the first month and was less than 10% by 2 months of age. Infant serum levels of S-fluoxetine and S-norfluoxetine were about 3 times as high as the R-isomers during the first 2 months. Only norfluoxetine could be detected after this time and the S- to R-isomer ratio fell to about 1.4.[8]
The ratio of infant to maternal serum levels of fluoxetine plus S-norfluoxetine dropped rapidly during the first month and was less than 10% by 2 months of age. Infant serum levels of S-fluoxetine and S-norfluoxetine were about 3 times as high as the R-isomers during the first 2 months. Only norfluoxetine could be detected after this time and the S- to R-isomer ratio fell to about 1.4.[8]
Effects in Breastfed Infants
Colic, decreased sleep, vomiting and watery stools occurred in a 6-day-old breastfed infant probably caused by maternal fluoxetine.[16] Two other reports of colic in breastfed infants, a 1.76-month-old and a 2-month-old, were possibly caused by fluoxetine in breastmilk. The older of the two also exhibited hyperactivity.[9]
Another case of possible increased irritability in a 3-month-old was noted by a pediatrician observer, who was the infant’s father. However, the mother and the infant’s pediatrician disagreed.[19]
Occurrence of hyperglycemia and glycosuria in a 5-month-old, possibly from fluoxetine in breastmilk was reported to the Australian Adverse Drug Reaction Advisory Committee. [20]
[20]
A 3-day-old breastfed infant was difficult to arouse, ceased rooting behavior, decreased nursing, and was moaning and grunting. Although the infant had been exposed in utero and was somewhat drowsy during the first 2 days of life, symptoms became worse after the mother's milk came in on day 3. These effects were probably caused by fluoxetine in breastmilk.[17]
Possible drug-induced seizure-like activity and cyanosis occurred in a breastfed 3-week-old breastfed infant whose mother was taking fluoxetine, carbamazepine and buspirone during pregnancy and breastfeeding.[21]
One observational report of 4 infants found no apparent neurological abnormalities following exposure to fluoxetine in milk for 12 to 52 weeks.[22]
A retrospective, case-control, cohort study compared the weights of the infants of mothers who took fluoxetine during pregnancy and breastfed for at least 2 weeks postpartum to the infants of mothers who took fluoxetine during pregnancy and did not breastfeed. Compared to controls, decreased weight gain occurred among the 26 infants exposed postpartum to fluoxetine in breastmilk, although the weights were still in the normal range.[23]
Compared to controls, decreased weight gain occurred among the 26 infants exposed postpartum to fluoxetine in breastmilk, although the weights were still in the normal range.[23]
A prospective study of 51 nursing women taking fluoxetine and 63 nursing women who took no fluoxetine found no effect on weight gain, but reported a greater frequency of unspecified side effects in the infants of mothers who took fluoxetine.[24] This study's results have been reported only in abstract form, so some details are lacking.
In a prospective study of 40 women who took fluoxetine throughout pregnancy, 21 breastfed their infants (extent and duration not stated). Testing of the infants at 15 to 71 months of age found no differences in cognitive, language or temperament measurements between infants who were breastfed and those who were not.[25]
In a study comparing the 31 infants of depressed mothers who took an SSRI during pregnancy for major depression with 13 infants of depressed mothers who did not take an SSRI, mental development and most motor development in both groups was normal at follow-up averaging 12. 9 months. Three of the treated mothers took fluoxetine in doses averaging 23.3 mg daily for an average of 3 months while breastfeeding their infants. Psychomotor development was slightly delayed compared to controls, but the contribution of breastfeeding to abnormal development could not be determined.[26]
9 months. Three of the treated mothers took fluoxetine in doses averaging 23.3 mg daily for an average of 3 months while breastfeeding their infants. Psychomotor development was slightly delayed compared to controls, but the contribution of breastfeeding to abnormal development could not be determined.[26]
Platelet serotonin levels were measured in 11 mothers and their breastfed infants after 4 to 12 weeks of fluoxetine therapy. Platelets and neurons both have the same serotonin transporter, so this effect on platelet serotonin might indicate potential effects on the nervous system of some breastfed infants. Maternal fluoxetine dosages ranged from 20 to 40 mg daily. Ten of the infants were under 6 months of age and 4 were under 3 months of age at the start of therapy; 6 were exclusively breastfed. Although maternal platelet serotonin levels were decreased from 157 mcg/L to 23 mcg/L by fluoxetine therapy, average infant serotonin levels were 217 mcg/L before and 230 mcg/L after maternal therapy. These findings indicate that the amount of fluoxetine ingested by the infants was not sufficient to affect serotonin transport in platelets in most breastfed infants. However, 3 infants experienced drops in platelet serotonin of 13, 24 and 60%, respectively. The latter infant was the only one with measurable fluoxetine plasma levels as well as norfluoxetine, but the infant had no discernible adverse effects. One other infant had a delay in motor development at 24 weeks, but had normal mental development; 6 other infants were within 1 standard deviation of normal in both measures when tested between 24 and 56 weeks of age.[18]
These findings indicate that the amount of fluoxetine ingested by the infants was not sufficient to affect serotonin transport in platelets in most breastfed infants. However, 3 infants experienced drops in platelet serotonin of 13, 24 and 60%, respectively. The latter infant was the only one with measurable fluoxetine plasma levels as well as norfluoxetine, but the infant had no discernible adverse effects. One other infant had a delay in motor development at 24 weeks, but had normal mental development; 6 other infants were within 1 standard deviation of normal in both measures when tested between 24 and 56 weeks of age.[18]
Twenty-nine mothers who took fluoxetine in an average dosage of 34.6 mg daily for depression or anxiety starting no later than 4 weeks postpartum, breastfed their infants exclusively for 4 months and at least 50% during months 5 and 6. Their infants had 6-month weight gains that were normal according to national growth standards and mothers reported no abnormal effects in their infants. [27]
[27]
One study of side effects of SSRI antidepressants in nursing mothers found no adverse reactions that required medical attention in one infant whose mother was taking fluoxetine. No specific information on maternal fluoxetine dosage, extent of breastfeeding or infant age was reported.[28]
Eleven infants who were breastfed (extent and duration not stated) during maternal use of fluoxetine for depression (n = 5) or panic disorder (n = 6) had normal weight gain at 12 months of age that was not significantly different from a control group of infants whose mothers took no psychotropic medications. Neurologic development was also normal at 12 months of age.[5]
In 1 breastfed (extent not stated) infant aged 11 weeks whose mother was taking fluoxetine 20 mg daily, no adverse reactions were noted clinically at the time of the study.[6]
A small study compared the reaction to pain in infants of depressed mothers who had taken an SSRI during pregnancy alone or during pregnancy and nursing to a control group of unexposed infants of nondepressed mothers. Infants exposed to an SSRI either prenatally alone or prenatally and postnatally via breastmilk had blunted responses to pain compared to control infants. Seven of the 30 infants were exposed to fluoxetine. Because there was no control group of depressed, nonmedicated mothers, an effect due to maternal behavior caused by depression could not be ruled out. The authors stressed that these findings did not warrant avoiding drug treatment of depression during pregnancy or avoiding breastfeeding during SSRI treatment.[7]
Infants exposed to an SSRI either prenatally alone or prenatally and postnatally via breastmilk had blunted responses to pain compared to control infants. Seven of the 30 infants were exposed to fluoxetine. Because there was no control group of depressed, nonmedicated mothers, an effect due to maternal behavior caused by depression could not be ruled out. The authors stressed that these findings did not warrant avoiding drug treatment of depression during pregnancy or avoiding breastfeeding during SSRI treatment.[7]
An infant was born to a mother taking fluoxetine 40 mg daily, oxycodone 20 mg 3 times daily, and quetiapine 400 mg daily. The infant was breastfed 6 to 7 times daily and was receiving 120 mcg of oral morphine 3 times daily for opiate withdrawal. Upon examination at 3 months of age, the infant's weight was at the 25th percentile for age, having been at the 50th percentile at birth. The authors attributed the weight loss to opiate withdrawal. The infant's Denver developmental score was equal to his chronological age. [29]
[29]
An uncontrolled online survey compiled data on 930 mothers who nursed their infants while taking an antidepressant. Infant drug discontinuation symptoms (e.g., irritability, low body temperature, uncontrollable crying, eating and sleeping disorders) were reported in about 10% of infants. Mothers who took antidepressants only during breastfeeding were much less likely to notice symptoms of drug discontinuation in their infants than those who took the drug in pregnancy and lactation.[30]
A cohort of 247 infants exposed to an antidepressant in utero during the third trimester of pregnancy were assessed for poor neonatal adaptation (PNA). Of the 247 infants, 154 developed PNA. Infants who were exclusively given formula had about 3 times the risk of developing PNA as those who were exclusively or partially breastfed. Fifteen of the infants were exposed to fluoxetine in utero.[31]
A late preterm infant was born to a mother who took fluoxetine 60 mg daily throughout pregnancy and during exclusive breastfeeding. At 7 days of age, the infant was found to be having jerking movements, with hypertonia and hyperreflexia as well as tachypnea and compensated metabolic acidosis. The infant's Finnegan scores were the range of 7 to 10. On day 8 of life, the infant had a serum fluoxetine level of 120 mcg/L, which is similar to therapeutic adult levels. Breastfeeding was discontinued and after 5 days of formula feeding the infant's Finnegan scores had decreased to a range of 3 to 6. After 10 days of formula, most symptoms had subsided. At 3 months of age, the infant was growing and developing normally. The infant's symptoms were attributed to serotonin syndrome caused by the high levels of fluoxetine rather than to withdrawal.[32] The reaction was probably caused by fluoxetine and breastfeeding might have contributed to maintaining the high fluoxetine levels after birth.
At 7 days of age, the infant was found to be having jerking movements, with hypertonia and hyperreflexia as well as tachypnea and compensated metabolic acidosis. The infant's Finnegan scores were the range of 7 to 10. On day 8 of life, the infant had a serum fluoxetine level of 120 mcg/L, which is similar to therapeutic adult levels. Breastfeeding was discontinued and after 5 days of formula feeding the infant's Finnegan scores had decreased to a range of 3 to 6. After 10 days of formula, most symptoms had subsided. At 3 months of age, the infant was growing and developing normally. The infant's symptoms were attributed to serotonin syndrome caused by the high levels of fluoxetine rather than to withdrawal.[32] The reaction was probably caused by fluoxetine and breastfeeding might have contributed to maintaining the high fluoxetine levels after birth.
A woman with narcolepsy took sodium oxybate 4 grams each night at 10 pm and 2 am as well as fluoxetine 20 mg and cetirizine 5 mg daily throughout pregnancy and postpartum. She breastfed her infant except for 4 hours after the 10 pm oxybate dose and 4 hours after the 2 am dose. She either pumped breastmilk or breastfed her infant just before each dose of oxybate. The infant was exclusively breastfed or breastmilk fed for 6 months when solids were introduced. The infant was evaluated at 2, 4 and 6 months with the Ages and Stages Questionnaires, which were withing the normal range as were the infant's growth and pediatrician's clinical impressions regarding the infant's growth and development.[33]
She breastfed her infant except for 4 hours after the 10 pm oxybate dose and 4 hours after the 2 am dose. She either pumped breastmilk or breastfed her infant just before each dose of oxybate. The infant was exclusively breastfed or breastmilk fed for 6 months when solids were introduced. The infant was evaluated at 2, 4 and 6 months with the Ages and Stages Questionnaires, which were withing the normal range as were the infant's growth and pediatrician's clinical impressions regarding the infant's growth and development.[33]
Two women were treated with fluoxetine 20 mg daily during the third trimester of pregnancy and during breastfeeding. Pediatric evaluations including neurologic assessments and brain ultrasound were conducted during the first 24 hours postpartum. Further follow-up was conducted at 6 or more months of age. Infant clinical status was comparable to unexposed infants from the same pediatric department.[15]
Effects on Lactation and Breastmilk
Fluoxetine has caused increased prolactin levels and galactorrhea in nonpregnant, nonnursing patients. [34-42] Euprolactinemic galactorrhea has also been reported.[43] In a study of cases of hyperprolactinemia and its symptoms (e.g., gynecomastia) reported to a French pharmacovigilance center, fluoxetine was found to have a 3.6-fold increased risk of causing hyperprolactinemia compared to other drugs.[38] Preliminary animal and in vitro studies found that fluoxetine may have some estrogenic activity.[39] The prolactin level in a mother with established lactation may not affect her ability to breastfeed.
[34-42] Euprolactinemic galactorrhea has also been reported.[43] In a study of cases of hyperprolactinemia and its symptoms (e.g., gynecomastia) reported to a French pharmacovigilance center, fluoxetine was found to have a 3.6-fold increased risk of causing hyperprolactinemia compared to other drugs.[38] Preliminary animal and in vitro studies found that fluoxetine may have some estrogenic activity.[39] The prolactin level in a mother with established lactation may not affect her ability to breastfeed.
In a small prospective study, 8 primiparous women who were taking a serotonin reuptake inhibitor (SRI; 3 taking fluoxetine and 1 each taking citalopram, duloxetine, escitalopram, paroxetine or sertraline) were compared to 423 mothers who were not taking an SRI. Mothers taking an SRI had an onset of milk secretory activation (lactogenesis II) that was delayed by an average of 16.7 hours compared to controls (85.8 hours postpartum in the SRI-treated mothers and 69.1 h in the untreated mothers), which doubled the risk of delayed feeding behavior compared to the untreated group. However, the delay in lactogenesis II may not be clinically important, since there was no statistically significant difference between the groups in the percentage of mothers experiencing feeding difficulties after day 4 postpartum.[40]
However, the delay in lactogenesis II may not be clinically important, since there was no statistically significant difference between the groups in the percentage of mothers experiencing feeding difficulties after day 4 postpartum.[40]
A case control study compared the rate of predominant breastfeeding at 2 weeks postpartum in mothers who took an SSRI antidepressant throughout pregnancy and at delivery (n = 167) or an SSRI during pregnancy only (n = 117) to a control group of mothers who took no antidepressants (n = 182). Among the two groups who had taken an SSRI, 33 took citalopram, 18 took escitalopram, 63 took fluoxetine, 2 took fluvoxamine, 78 took paroxetine, and 87 took sertraline. Among the women who took an SSRI, the breastfeeding rate at 2 weeks postpartum was 27% to 33% lower than mother who did not take antidepressants, with no statistical difference in breastfeeding rates between the SSRI-exposed groups.[41]
An observational study looked at outcomes of 2859 women who took an antidepressant during the 2 years prior to pregnancy. Compared to women who did not take an antidepressant during pregnancy, mothers who took an antidepressant during all 3 trimesters of pregnancy were 37% less likely to be breastfeeding upon hospital discharge. Mothers who took an antidepressant only during the third trimester were 75% less likely to be breastfeeding at discharge. Those who took an antidepressant only during the first and second trimesters did not have a reduced likelihood of breastfeeding at discharge.[44] The antidepressants used by the mothers were not specified.
Compared to women who did not take an antidepressant during pregnancy, mothers who took an antidepressant during all 3 trimesters of pregnancy were 37% less likely to be breastfeeding upon hospital discharge. Mothers who took an antidepressant only during the third trimester were 75% less likely to be breastfeeding at discharge. Those who took an antidepressant only during the first and second trimesters did not have a reduced likelihood of breastfeeding at discharge.[44] The antidepressants used by the mothers were not specified.
A retrospective cohort study of hospital electronic medical records from 2001 to 2008 compared women who had been dispensed an antidepressant during late gestation (n = 575; fluoxetine n = 21) to those who had a psychiatric illness but did not receive an antidepressant (n = 1552) and mothers who did not have a psychiatric diagnosis (n = 30,535). Women who received an antidepressant were 37% less likely to be breastfeeding at discharge than women without a psychiatric diagnosis, but no less likely to be breastfeeding than untreated mothers with a psychiatric diagnosis. [45]
[45]
In a study of 80,882 Norwegian mother-infant pairs from 1999 to 2008, new postpartum antidepressant use was reported by 392 women and 201 reported that they continued antidepressants from pregnancy. Compared with the unexposed comparison group, late pregnancy antidepressant use was associated with a 7% reduced likelihood of breastfeeding initiation, but with no effect on breastfeeding duration or exclusivity. Compared with the unexposed comparison group, new or restarted antidepressant use was associated with a 63% reduced likelihood of predominant, and a 51% reduced likelihood of any breastfeeding at 6 months, as well as a 2.6-fold increased risk of abrupt breastfeeding discontinuation. Specific antidepressants were not mentioned.[46]
Alternate Drugs to Consider
Nortriptyline, Paroxetine, Sertraline
References
- 1.
Larsen ER, Damkier P, Pedersen LH, et al. Use of psychotropic drugs during pregnancy and breast-feeding. Acta Psychiatr Scand Suppl.
 2015;132 Suppl 445:1–28. [PubMed: 26344706]
2015;132 Suppl 445:1–28. [PubMed: 26344706]- 2.
Larsen ER, Damkier P, Pedersen LH, et al. Use of psychotropic drugs during pregnancy and breast-feeding. Acta Psychiatr Scand Suppl. 2015:1–28. [PubMed: 26344706]
- 3.
Grzeskowiak LE, Leggett C, Costi L, et al. Impact of serotonin reuptake inhibitor use on breast milk supply in mothers of preterm infants: A retrospective cohort study. Br J Clin Pharmacol. 2018;84:1373–9. [PMC free article: PMC5980248] [PubMed: 29522259]
- 4.
Weissman AM, Levy BT, Hartz AJ, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. 2004;161:1066–78. [PubMed: 15169695]
- 5.
Heikkinen T, Ekblad U, Palo P, et al. Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin Pharmacol Ther. 2003;73:330–7. [PubMed: 12709723]
- 6.
Berle JØ, Steen VM, Aamo TO, et al. Breastfeeding during maternal antidepressant treatment with serotonin reuptake inhibitors: Infant exposure, clinical symptoms, and cytochrome P450 genotypes.
 J Clin Psychiatry. 2004;65:1228–34. [PubMed: 15367050]
J Clin Psychiatry. 2004;65:1228–34. [PubMed: 15367050]- 7.
Oberlander TF, Grunau RE, Fitzgerald C, et al. Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115:411–25. [PubMed: 15687451]
- 8.
Kim J, Riggs KW, Misri S, et al. Stereoselective disposition of fluoxetine and norfluoxetine during pregnancy and breast-feeding. Br J Clin Pharmacol. 2006;61:155–63. [PMC free article: PMC1885002] [PubMed: 16433870]
- 9.
Kristensen JH, Ilett KF, Hackett LP, et al. Distribution and excretion of fluoxetine and norfluoxetine in human milk. Br J Clin Pharmacol. 1999;48:521–7. [PMC free article: PMC2014386] [PubMed: 10583022]
- 10.
Taddio A, Ito S, Koren G. Excretion of fluoxetine and its metabolite, norfluoxetine, in human breast milk. J Clin Pharmacol. 1996;36:42–7. [PubMed: 8932542]
- 11.
Tanoshima R, Bournissen FG, Tanigawara Y, et al.
 Population PK modelling and simulation based on fluoxetine and norfluoxetine concentrations in milk: A milk concentration-based prediction model. Br J Clin Pharmacol. 2014;78:918–28. [PMC free article: PMC4239985] [PubMed: 24773313]
Population PK modelling and simulation based on fluoxetine and norfluoxetine concentrations in milk: A milk concentration-based prediction model. Br J Clin Pharmacol. 2014;78:918–28. [PMC free article: PMC4239985] [PubMed: 24773313]- 12.
Salazar FR, D'Avila FB, de Oliveira MH, et al. Development and validation of a bioanalytical method for five antidepressants in human milk by LC-MS. J Pharm Biomed Anal. 2016;129:502–8. [PubMed: 27497651]
- 13.
Weisskopf E, Panchaud A, Nguyen KA, et al. Simultaneous determination of selective serotonin reuptake inhibitors and their main metabolites in human breast milk by liquid chromatography-electrospray mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1057:101–9. [PubMed: 28511118]
- 14.
Lopes BR, Cassiano NM, Carvalho DM, et al. Simultaneous quantification of fluoxetine and norfluoxetine in colostrum and mature human milk using a 2-dimensional liquid chromatography-tandem mass spectrometry system.
 J Pharm Biomed Anal. 2018;150:362–7. [PubMed: 29287263]
J Pharm Biomed Anal. 2018;150:362–7. [PubMed: 29287263]- 15.
Pogliani L, Baldelli S, Cattaneo D, et al. Selective serotonin reuptake inhibitors passage into human milk of lactating women. J Matern Fetal Neonatal Med. 2019;32:3020–5. [PubMed: 29557689]
- 16.
Lester BM, Cucca J, Andreozzi L, et al. Possible association between fluoxetine hydrochloride and colic in an infant. J Am Acad Child Adolesc Psychiatry. 1993;32:1253–5. [PubMed: 8282672]
- 17.
Hale TW, Shum S, Grossberg M. Fluoxetine toxicity in a breastfed infant. Clin Pediatr (Phila). 2001;40:681–4. [PubMed: 11771923]
- 18.
Epperson CN, Jatlow PI, Czarkowski K, et al. Maternal fluoxetine treatment in the postpartum period: effects on platelet serotonin and plasma drug levels in breastfeeding mother-infant pairs. Pediatrics. 2003;112:e425–9. [PubMed: 14595087]
- 19.
Isenberg KE. Excretion of fluoxetine in human breast milk.
 J Clin Psychiatry 1990;51:169. Letter. PMID: 2324084. [PubMed: 2324084]
J Clin Psychiatry 1990;51:169. Letter. PMID: 2324084. [PubMed: 2324084]- 20.
Rohan A. Drug distribution in human milk. Aust Prescr. 1997;20:84.
- 21.
Brent NB, Wisner KL. Fluoxetine and carbamazepine concentrations in a nursing mother/infant pair. Clin Pediatr (Phila). 1998;37:41–4. [PubMed: 9475699]
- 22.
Yoshida K, Smith B, Craggs M, et al. Fluoxetine in breast-milk and developmental outcome of breast-fed infants. Br J Psychiatry. 1998;172:175–8. [PubMed: 9519072]
- 23.
Chambers CD, Anderson PO, Thomas RG, et al. Weight gain in infants breastfed by mothers who take fluoxetine. Pediatrics. 1999;104:e61. [PubMed: 10545587]
- 24.
Moretti ME, Sharma A, Bar-Oz B, et al. Fluoxetine and its effects on the nursing infant: A prospective cohort. Clin Pharmacol Ther 1999;65:141. Abstract. doi: 10.1016/S0009-9236(99)80095-2. [CrossRef]
- 25.
Nulman I, Koren G. The safety of fluoxetine during pregnancy and lactation.
 Teratology. 1996;53:304–8. [PubMed: 8879088]
Teratology. 1996;53:304–8. [PubMed: 8879088]- 26.
Casper RC, Fleisher BE, Lee-Ancajas JC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–8. [PubMed: 12712058]
- 27.
Hendrick V, Smith LM, Hwang S, et al. Weight gain in breastfed infants of mothers taking antidepressant medications. J Clin Psychiatry. 2003;64:410–2. [PubMed: 12716242]
- 28.
Lee A, Woo J, Ito S. Frequency of infant adverse events that are associated with citalopram use during breast-feeding. Am J Obstet Gynecol. 2004;190:218–21. [PubMed: 14749663]
- 29.
Rampono J, Kristensen JH, Ilett KF, et al. Quetiapine and breast feeding. Ann Pharmacother. 2007;41:711–4. [PubMed: 17374621]
- 30.
Hale TW, Kendall-Tackett K, Cong Z, et al. Discontinuation syndrome in newborns whose mothers took antidepressants while pregnant or breastfeeding.
 Breastfeed Med. 2010;5:283–8. [PubMed: 20807106]
Breastfeed Med. 2010;5:283–8. [PubMed: 20807106]- 31.
Kieviet N, Hoppenbrouwers C, Dolman KM, et al. Risk factors for poor neonatal adaptation after exposure to antidepressants in utero. Acta Paediatr. 2015;104:384–91. [PubMed: 25559357]
- 32.
Morris R, Matthes J. Serotonin syndrome in a breast-fed neonate. BMJ Case Rep 2015;2015. PMID: 25948853. [PMC free article: PMC4434373] [PubMed: 25948853]
- 33.
Gashlin LZ, Sullo D, Lawrence RA, et al. Treatment of narcolepsy with sodium oxybate while breastfeeding: A case report. Breastfeed Med. 2016;11:261–3. [PMC free article: PMC4921898] [PubMed: 27057786]
- 34.
Arya DK, Taylor WS. Lactation associated with fluoxetine treatment. Aust N Z J Psychiatry 1995;29:697. Letter. PMID: 8825840. [PubMed: 8825840]
- 35.
Egberts AC, Meyboom RH, De Koning FH, et al. Non-puerperal lactation associated with antidepressant drug use. Br J Clin Pharmacol.
 1997;44:277–81. [PMC free article: PMC2042834] [PubMed: 9296322]
1997;44:277–81. [PMC free article: PMC2042834] [PubMed: 9296322]- 36.
Iancu I, Ratzoni G, Weitzman A, et al. More fluoxetine experience. J Am Acad Child Adolesc Psychiatry 1992;31:755-6. Letter. PMID: 1644743. [PubMed: 1644743]
- 37.
Peterson MC. Reversible galactorrhea and prolactin elevation related to fluoxetine use. Mayo Clin Proc. 2001;76:215–6. [PubMed: 11213313]
- 38.
Trenque T, Herlem E, Auriche P, et al. Serotonin reuptake inhibitors and hyperprolactinaemia: A case/non-case study in the French pharmacovigilance database. Drug Saf. 2011;34:1161–6. [PubMed: 22077504]
- 39.
Müller JC, Imazaki PH, Boareto AC, et al. In vivo and in vitro estrogenic activity of the antidepressant fluoxetine. Reprod Toxicol. 2012;34:80–5. [PubMed: 22522098]
- 40.
Marshall AM, Nommsen-Rivers LA, Hernandez LL, et al. Serotonin transport and metabolism in the mammary gland modulates secretory activation and involution.
 J Clin Endocrinol Metab. 2010;95:837–46. [PMC free article: PMC2840848] [PubMed: 19965920]
J Clin Endocrinol Metab. 2010;95:837–46. [PMC free article: PMC2840848] [PubMed: 19965920]- 41.
Gorman JR, Kao K, Chambers CD. Breastfeeding among women exposed to antidepressants during pregnancy. J Hum Lact. 2012;28:181–8. [PubMed: 22344850]
- 42.
Suthar N, Pareek V, Nebhinani N, et al. Galactorrhea with antidepressants: A case series. Indian J Psychiatry. 2018;60:145–6. [PMC free article: PMC5914246] [PubMed: 29736080]
- 43.
Kaba D, Oner O. Galactorrhea after selective serotonin reuptake inhibitor use in an adolescent girl: A case report. J Clin Psychopharmacol. 2017;37:374–6. [PubMed: 28383361]
- 44.
Venkatesh KK, Castro VM, Perlis RH, et al. Impact of antidepressant treatment during pregnancy on obstetric outcomes among women previously treated for depression: An observational cohort study. J Perinatol. 2017;37:1003–9. [PubMed: 28682318]
- 45.
Leggett C, Costi L, Morrison JL, et al.
 Antidepressant use in late gestation and breastfeeding rates at discharge from hospital. J Hum Lact. 2017;33:701–9. [PubMed: 28984528]
Antidepressant use in late gestation and breastfeeding rates at discharge from hospital. J Hum Lact. 2017;33:701–9. [PubMed: 28984528]- 46.
Grzeskowiak LE, Saha MR, Nordeng H, et al. Perinatal antidepressant use and breastfeeding outcomes: Findings from the Norwegian Mother, Father and Child Cohort Study. Acta Obstet Gynecol Scand. 2022;101:344–54. [PMC free article: PMC9564556] [PubMed: 35170756]
| 💊 Ingredients of Fluoxetine ✅ Use of Fluoxetine Save Search for analogues Interaction Description of the active ingredients of the preparation fluoxetine (Fluoxetine) The scientific information provided is general and cannot be used to make decisions. Update date: 2020.06.09 Marketing authorization holder:BIOCOM, JSC (Russia) ATX code: N06AB03 (Fluoxetine) Active substance: fluoxetine (fluoxetine) Rec.INN WHO registered Dosage form
Release form, packaging and composition Fluoxetine 10 pcs. - cellular contour packings (2) - packs of cardboard. Clinical and pharmacological group: Antidepressant Pharmacotherapeutic group: Antidepressant Pharmacological action Antidepressant, propylamine derivative. The mechanism of action is associated with selective blockade of neuronal reuptake of serotonin in the CNS. Fluoxetine is a weak antagonist of cholino-, adreno- and histamine receptors. PharmacokineticsAbsorbed from the gastrointestinal tract. Weakly metabolized during the "first pass" through the liver. Eating does not affect the degree of absorption, although it may slow down its rate. C max in plasma is achieved after 6-8 hours. C ss in plasma is achieved only after continuous administration for several weeks. Protein binding 94.5%. Easily penetrates through the BBB. It is metabolized in the liver by demethylation to form the main active metabolite of norfluoxetine. T 1/2 fluoxetine is 2-3 days, norfluoxetine is 7-9 days. Excreted by the kidneys 80% and through the intestines - about 15%. Indications of the active substances of the drug fluoxetineDepression of various origins, obsessive-compulsive disorders, bulimic neurosis. Open list of ICD-10 codes
Dosage regimen The method of administration and dosing regimen of a particular drug depends on its form of release and other factors. Initial dose - 20 mg 1 time / day in the morning; if necessary, the dose can be increased after 3-4 weeks. The frequency of admission is 2-3 times / day. The maximum daily oral dose of for adults is 80 mg. Side effectsFrom the side of the central nervous system: possible anxiety, tremor, nervousness, drowsiness, headache, sleep disturbances. From the digestive system: possible diarrhea, nausea. From the side of metabolism: increased sweating, hypoglycemia, hyponatremia (especially in elderly patients and with hypovolemia) are possible. From the reproductive system: decreased libido. Allergic reactions: possible skin rash, itching. Other: pain in the joints and muscles, shortness of breath, fever. Contraindications for useGlaucoma, bladder atony, severe renal dysfunction, benign prostatic hyperplasia, concomitant administration of MAO inhibitors, convulsive syndrome of various origins, epilepsy, pregnancy, lactation, hypersensitivity to fluoxetine. Use in pregnancy and lactationContraindicated in pregnancy and lactation. Use in hepatic impairmentUse with extreme caution in patients with hepatic impairment. Use in impaired renal functionContraindicated in severe renal impairment. Use with extreme caution in patients with moderate and mild renal impairment. Use in childrenThe safety of fluoxetine in children has not been established. Use in elderly patientsElderly patients require dosage adjustment. Special instructions Use with extreme caution in patients with impaired liver and kidney function, with a history of epileptic seizures, cardiovascular diseases. In patients with diabetes mellitus, changes in blood glucose levels are possible, which requires correction of the dosing regimen of hypoglycemic drugs. When used in debilitated patients while taking fluoxetine, the likelihood of developing epileptic seizures increases. With the simultaneous use of fluoxetine and electroconvulsive therapy, prolonged epileptic seizures may develop. Fluoxetine can be used no earlier than 14 days after discontinuation of MAO inhibitors. The period after the abolition of fluoxetine before the start of therapy with MAO inhibitors should be at least 5 weeks. Elderly patients require dosage adjustment. The safety of fluoxetine in children has not been established. Do not drink alcohol during treatment. Influence on the ability to drive vehicles and mechanisms During the period of treatment, one should refrain from potentially hazardous activities that require increased attention and rapid psychomotor reactions. Drug interactionsWhen used simultaneously with drugs that have a depressant effect on the central nervous system, with ethanol, a significant increase in the inhibitory effect on the central nervous system, as well as an increase in the likelihood of convulsions, is possible. With simultaneous use with MAO inhibitors, furazolidone, procarbazine, tryptophan, serotonin syndrome may develop (confusion, hypomania, restlessness, agitation, convulsions, dysarthria, hypertensive crisis, chills, tremor, nausea, vomiting, diarrhea). With simultaneous use, fluoxetine inhibits the metabolism of tricyclic and tetracyclic antidepressants, trazodone, carbamazepine, diazepam, metoprolol, terfenadine, phenytoin, which leads to an increase in their concentration in blood serum, an increase in their therapeutic and side effects. With simultaneous use, inhibition of the biotransformation of drugs metabolized with the participation of the CYP2D6 isoenzyme is possible. When used simultaneously with hypoglycemic agents, their action may be enhanced. There have been reports of increased effects of warfarin when co-administered with fluoxetine. When used simultaneously with haloperidol, fluphenazine, maprotiline, metoclopramide, perphenazine, periciazine, pimozide, risperidone, sulpiride, trifluoperazine, cases of extrapyramidal symptoms and dystonia have been described; with dextromethorphan - a case of the development of hallucinations is described; with digoxin - a case of increasing the concentration of digoxin in the blood plasma. When used simultaneously with lithium salts, an increase or decrease in the concentration of lithium in the blood plasma is possible. With simultaneous use, an increase in the concentration of imipramine or desipramine in the blood plasma by 2-10 times is possible (may persist for 3 weeks after fluoxetine is discontinued). When used simultaneously with propofol, a case has been described in which spontaneous movements were observed; with phenylpropanolamine - a case is described in which dizziness, weight loss, hyperactivity were observed. Co-administration may enhance the effects of flecainide, mexiletine, propafenone, thioridazine, zuclopenthixol. Keep If you want to place a link to the description of this drug - use this code Fluoxetine . Description of the drug in the reference book Vidal. |
Fluoxetine and breastfeeding. Can fluoxetine be taken while breastfeeding?
Fluoxetine and breastfeeding. Can fluoxetine be taken while breastfeeding? | E-lactationCan Fluoxetine be taken while breastfeeding? What are the alternatives to Fluoxetine?
July 1, 2015 (Low risk)
Higher excretion into breast milk than other antidepressants. The active metabolite, called norfluoxetine, has a longer half-life (4 to 16 days). As with other antidepressants, hyperprolactinemia and galactorrhea may be induced.
Several cases of colic, irritability, insomnia, anorexia and weight loss have been described. However, most of the reported cases failed to show an effect of harm beyond the neonatal period. Extensive experience with fluoxatin has not adversely affected neonatal weight gain and neurological development in the short or long term.
Most problems appeared in the early neonatal period, either in newborns or premature babies whose mothers were on fluoxetine during pregnancy. Stands or switches to other medicines either on some days before delivery or in the first month after delivery. The same considerations should be made in case of prematurity, however, if necessary, treatment should be continued.
Low risk
Moderately safe. Read the comment carefully.
High risk
Dangerous. Use a less risky alternative. Read the comment.
Very high risk
Very dangerous. High risk of stopping breastfeeding.
Synonyms
- FLUOXETINE HYDROCHLORIDE
- Lilly-103472
- LY -10140 GARECISTIONS OPENSIONS IN THE POODUCTIONS)0256
- FLUXEN®
- FLUOXETINE
- FLUOXETINE HYDROCHLORIDE
- Sriraman NK, Melvin K, Meltzer-Brody S.
ABM Antidepressant Clinical Protocol #18: Use of Breast Antidepressants Clinical Protocol #18: Use of Breast Antidepressants. Breastfeed Med. 2015 annotation Full text (source link) Full text (link on our server)
- Nebhinani N. Sertraline-induced galactorrhea: case report and review of cases reported with other SSRIs. Gen Hosp Psychiatry. 2013 annotation
- Rowe H, Baker T, Hale TW.
Maternal medication, drug use, and breastfeeding. Pediatric Clin North Am. 2013 Feb;60(1):275-94. 2013 annotation
- Sachs HC; committee on drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013 annotation Full text (source link) Full text (link on our server)
- Nielsen RE, Damkier P.
Pharmacological treatment of unipolar depression during pregnancy and breast-feeding--a clinical overview. Nord J Psychiatry. 2012 annotation
- Gorman JR, Kao K, Chambers CD. Breastfeeding among women exposed to antidepressants during pregnancy. J Hum Lact. 2012 annotation
- Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A.
Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011 annotation
- Patil AS, Kuller JA, Rhee EH. Antidepressants in pregnancy: a review of commonly prescribed medications. Obstet Gynecol Surv. 2011 annotation
- Trenque T, Herlem E, Auriche P, Dramé M.
Serotonin reuptake inhibitors and hyperprolactinaemia: a case/non-case study in the French pharmacovigilance database. Drug Saf. 2011 annotation
- Berle JO, Spigset O. Antidepressant Use During Breastfeeding. Curr Womens Health Rev. 2011 annotation Full text (source link) Full text (link on our server)
- Amir LH, Pirotta MV, Raval M.
Breastfeeding--evidence based guidelines for the use of medicines. Aust Fam Physician. 2011 annotation Full text (source link) Full text (link on our server)
- Hale TW, Kendall-Tackett K, Cong Z, Votta R, McCurdy F. Discontinuation syndrome in newborns whose mothers took antidepressants while pregnant or breastfeeding. Breastfeed Med. 2010 annotation
- Lanza di Scalea T, Wisner KL.
Antidepressant medication use during breastfeeding. Clin Obstet Gynecol. 2009 annotation Full text (source link) Full text (link on our server)
- Field T. Breastfeeding and antidepressants. Infant Behav Dev. 2008 annotation Full text (source link) Full text (link on our server)
- Academy of Breastfeeding Medicine Protocol Committee.
ABM clinical protocol #18: use of antidepressants in nursing mothers. Breastfeed Med. 2008 annotation Full text (source link) Full text (link on our server)
- Papakostas GI, Miller KK, Petersen T, Sklarsky KG, Hilliker SE, Klibanski A, Fava M. Serum prolactin levels among outpatients with major depressive disorder during the acute phase of treatment with fluoxetine. J Clin Psychiatry. 2006 annotation
- Oberlander TF, Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, Riggs W.
Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005 annotation
- Gentile S. The safety of newer antidepressants in pregnancy and breastfeeding. Drug Saf. 2005 annotation
- Berle JØ, Steen VM, Aamo TO, Breilid H, Zahlsen K, Spigset O.
Breastfeeding during maternal antidepressant treatment with serotonin reuptake inhibitors: infant exposure, clinical symptoms, and cytochrome p450 genotypes. J Clin Psychiatry. 2004 annotation
- Hostetter AL, Stowe ZN, Cox M, Ritchie JC. A novel system for the determination of antidepressant concentrations in human breast milk. Ther Drug Monitor. 2004 annotation
- Rubin ET, Lee A, Ito S.
When breastfeeding mothers need CNS-acting drugs. Can J Clin Pharmacol. 2004 annotation
- Weissman AM, Levy BT, Hartz AJ, Bentler S, Donohue M, Ellingrod VL, Wisner KL. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. 2004 annotation Full text (source link) Full text (link on our server)
- Heikkinen T, Ekblad U, Palo P, Laine K.
Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin Pharmacol Ther. 2003 annotation
- Hendrick V, Smith LM, Hwang S, Altshuler LL, Haynes D. Weight gain in breastfed infants of mothers taking antidepressant medications. J Clin Psychiatry. 2003 annotation
- Epperson CN, Jatlow PI, Czarkowski K, Anderson GM.
Maternal fluoxetine treatment in the postpartum period: effects on platelet serotonin and plasma drug levels in breastfeeding mother-infant pairs. Pediatrics. 2003 annotation Full text (link on our server)
- Gjerdingen D. The effectiveness of various postpartum depression treatments and the impact of antidepressant drugs on nursing infants. J Am Board Fam Pract. 2003 annotation Full text (source link) Full text (link on our server)
- Suri R, Stowe ZN, Hendrick V, Hostetter A, Widawski M, Altshuler LL.
Estimates of nursing infant daily dose of fluoxetine through breast milk. Biol Psychiatry. 2002 annotation
- Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, Koren G. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry. 2002 annotation
- Wisner KL, Parry BL, Piontek CM.
Clinical practice. Postpartum depression. N Engl J Med. 2002 annotation
- Peterson MC. Reversible galactorrhea and prolactin elevation related to fluoxetine use. Mayo Clin Proc. 2001 annotation Full text (link on our server)
- Pomp EF, Gedde-Dahl A.
Fluoksetin – trygt under graviditet og amming. \ [Fluoxetine--safe during pregnancy and breast feeding]. Tidsskr Nor Laegeforen. 2001 annotation Full text (link on our server)
- Hendrick V, Stowe ZN, Altshuler LL, Mintz J, Hwang S, Hostetter A, Suri R, Leight K, Fukuchi A. Fluoxetine and norfluoxetine concentrations in nursing infants and breast milk. Biol Psychiatry. 2001 annotation
- Nordeng H, Bergsholm YK, Bøhler E, Spigset O.
Overgang av selektive serotoninreopptakshemmere til morsmelk \ [The transfer of selective serotonin reuptake inhibitors to human milk]. Tidsskr Nor Laegeforen. 2001 annotation Full text (source link) Full text (link on our server)
- Pomp EF, Gedde-Dahl A. [Fluoxetine--safe during pregnancy and breast feeding]. Tidsskr Nor Laegeforen. 2001 annotation
- Nordeng H, Lindemann R, Perminov KV, Reikvam A.
Neonatal withdrawal syndrome after in utero exposure to selective serotonin reuptake inhibitors. Acta Paediatr. 2001 annotation
- Nordeng H, Bergsholm YK, Bøhler E, Spigset O. [The transfer of selective serotonin reuptake inhibitors to human milk]. Tidsskr Nor Laegeforen. 2001 annotation
- Birnbaum CS, Cohen LS, Bailey JW, Grush LR, Robertson LM, Stowe ZN.
Serum concentrations of antidepressants and benzodiazepines in nursing infants: A case series. Pediatrics. 1999 annotation Full text (source link) Full text (link on our server)
- Chambers CD, Anderson PO, Thomas RG, Dick LM, Felix RJ, Johnson KA, Jones KL. Weight gain in infants breastfed by mothers who take fluoxetine. Pediatrics. 1999 annotation Full text (link on our server)
- Kristensen JH, Ilett KF, Hackett LP, Yapp P, Paech M, Begg EJ.
Distribution and excretion of fluoxetine and norfluoxetine in human milk. Br J Clin Pharmacol. 1999 annotation Full text (link on our server)
- Ohman R, Hägg S, Carleborg L, Spigset O. Excretion of paroxetine into breast milk. J Clin Psychiatry. 1999 annotation
- Brent NB, Wisner KL.
Fluoxetine and carbamazepine concentrations in a nursing mother/infant pair. Clin Pediatr (Phila). 1998 annotation
- Yoshida K, Smith B, Craggs M, Kumar RC. Fluoxetine in breast-milk and developmental outcome of breast-fed infants. Br J Psychiatry. 1998 annotation
- Egberts AC, Meyboom RH, De Koning FH, Bakker A, Leufkens HG.
Non-puerperal lactation associated with antidepressant drug use. Br J Clin Pharmacol. 1997 annotation Full text (source link) Full text (link on our server)
- Spigset O, Carieborg L, Ohman R, Norström A. Excretion of citalopram in breast milk. Br J Clin Pharmacol. 1997 annotation Full text (source link) Full text (link on our server)
- Håberg M, Matheson I.
[Antidepressive agents and breast feeding]. Tidsskr Nor Laegeforen. 1997 annotation
- Mammen O, Perel JM, Wheeler S. Antidepressants and breast-feeding. Am J Psychiatry. 1997 annotation Full text (source link) Full text (link on our server)
- Wisner KL, Perel JM, Findling RL.
Antidepressant treatment during breast-feeding. Am J Psychiatry. 1996 annotation
- Taddio A, Ito S, Koren G. Excretion of fluoxetine and its metabolite, norfluoxetine, in human breast milk. J Clin Pharmacol. 1996 annotation
- Duffull SB, Begg EJ, Ilett KF.
Fluoxetine distribution in human milk. J Clin Pharmacol. 1996 annotation
- Arya DK, Taylor W.S. Lactation associated with fluoxetine treatment. Aust N Z J Psychiatry. 1995 annotation
- Lester BM, Cucca J, Andreozzi L, Flanagan P, Oh W.
Possible association between fluoxetine hydrochloride and colic in an infant. J Am Acad Child Adolesc Psychiatry. 1993 annotation
- Burch KJ, Wells BG. Fluoxetine/norfluoxetine concentrations in human milk. Pediatrics. 1992 annotation
- [No authors listed] Can a woman breastfeed while taking Prozac? J Hum Lact.
Learn more
 decisions about the use of a particular drug.
decisions about the use of a particular drug.  04.10 - Indefinitely Re-registration date: 16.09.eleven
04.10 - Indefinitely Re-registration date: 16.09.eleven  Unlike most antidepressants, fluoxetine does not appear to cause a decrease in the functional activity of postsynaptic β-adrenergic receptors. Helps improve mood, reduces feelings of fear and tension, eliminates dysphoria. Does not cause sedation. When taken in average therapeutic doses, it practically does not affect the functions of the cardiovascular and other systems.
Unlike most antidepressants, fluoxetine does not appear to cause a decrease in the functional activity of postsynaptic β-adrenergic receptors. Helps improve mood, reduces feelings of fear and tension, eliminates dysphoria. Does not cause sedation. When taken in average therapeutic doses, it practically does not affect the functions of the cardiovascular and other systems. 
 The optimal dosage regimen is determined by the doctor. Compliance of the dosage form of a particular drug with indications for use and dosing regimen should be strictly observed.
The optimal dosage regimen is determined by the doctor. Compliance of the dosage form of a particular drug with indications for use and dosing regimen should be strictly observed.