Can topamax cause depression
How Topamax May Treat and Cause Depression
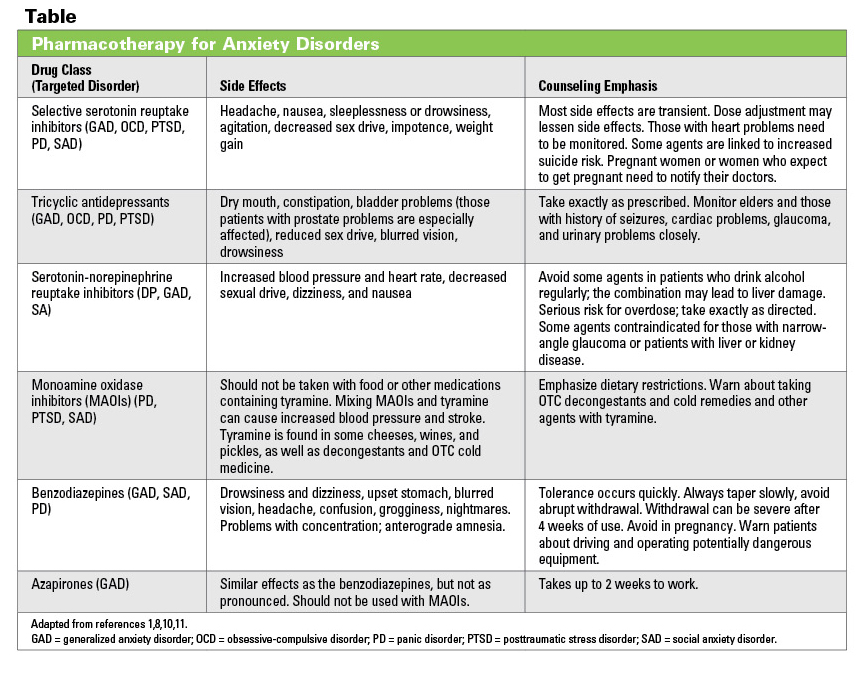
Topamax is the brand name for the drug topiramate. Topamax is approved to treat seizure disorders, like epilepsy, and to prevent migraine in adults.
Some people use Topamax to treat other conditions, like anxiety, depression, bipolar disorder, or post-traumatic stress disorder (PTSD), but Topamax is not approved by the Food and Drug Administration (FDA) for these purposes.
Though a few small studies have shown promise for using Topamax to treat depression or bipolar disorder with depression, there have been no large, peer-reviewed studies that show definitively that Topamax is safe and effective for these conditions.
In one small 2002 study of 16 women with treatment-resistant depression, 44 percent treated with Topamax reported improvement after 18 weeks. Carpenter L. (2002). Do obese depressed patients respond to topiramate? A retrospective chart review. https://www.ncbi.nlm.nih.gov/pubmed/12103474/
A more recent double-blind, placebo-controlled clinical trial was composed of 42 patients with major depressive disorder (MDD) who failed to respond to at least eight weeks of treatment with a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine, citalopram or sertraline. Mowla A, et al. (2011). Topiramate augmentation in patients with resistant major depressive disorder: A double-blind placebo-controlled clinical trial. DOI: 10.1016/j.pnpbp.2011.01.016
The study found that the participants taking Topamax in addition to their prescribed depression medication significantly improved depressed mood, suicidality, insomnia, agitation, and anxiety symptoms compared to those taking a placebo.
In another randomized, single-blind study, individuals with bipolar disorder in the depressive phase showed a significant improvement of symptoms in 56 percent of patients treated with topiramate.McIntyre RS, et al. (2002). Topiramate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. https://www.ncbi.nlm.nih.gov/pubmed/12180276/
This was compared to 59 percent of patients who received another common anti-depressant known as bupropion (Wellbutrin). However, like the other studies mentioned above, this study was small, including a total of 36 patients.
However, like the other studies mentioned above, this study was small, including a total of 36 patients.
Larger clinical trials will be needed to confirm Topamax’s use in treating depression or bipolar depression before the drug can be approved for this condition.
Still, some doctors may choose to prescribe Topamax off label. Your doctor may decide to do this if several other antidepressant or mood-stabilizing drugs fail to control your symptoms.
Since one of the side effects of Topamax is weight loss, a doctor may also decide to prescribe Topamax alongside another antidepressant as an adjunctive therapy to help offset any weight gain that may have been caused by the antidepressant.Mahmood S, et al. (2013). Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. DOI: 1097/JCP.0b013e31827cb2b7
There have been a few reports of Topamax causing or worsening depression in people taking it for other conditions, such as seizures, migraines, or bipolar disorder. Klufas A, et al. (2001). Letters to the editor: Topiramate-Induced Depression. https://ajp.psychiatryonline.org/doi/pdf/10.1176/appi.ajp.158.10.1736
Klufas A, et al. (2001). Letters to the editor: Topiramate-Induced Depression. https://ajp.psychiatryonline.org/doi/pdf/10.1176/appi.ajp.158.10.1736
Topamax may increase a person’s risk of experiencing suicidal thoughts or behavior (thinking about hurting or killing yourself). About 1 in every 500 people who took anticonvulsants like Topamax during clinical studies became suicidal.Topamax (topiramate) medication guide. (2018). http://www.janssenlabels.com/package-insert/product-patient-information/TOPAMAX-medication-guide.pdf
symptoms to report if you take topamax
- new depression or worsening of depression
- suicidal thoughts
- attempts to commit suicide
- new or worsening anxiety
- irritability
- trouble sleeping
- panic attacks
- an extreme increase in activity and talking (mania)
- withdrawing from friends and family
- unusual changes in mood or behavior
Topamax is a prescription drug that belongs to a class of drugs called anticonvulsants or antiepileptic drugs (AEDs). It’s described on its FDA label as a “sulfamate-substituted monosaccharide.”Topamax (topiramate) label. (2017). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020505s057_020844s048lbl.pdf
It’s described on its FDA label as a “sulfamate-substituted monosaccharide.”Topamax (topiramate) label. (2017). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020505s057_020844s048lbl.pdf
Topamax tablets come as 25 milligrams (mg), 50 mg, 100 mg, and 200 mg round tablets that are taken whole by mouth. The drug also comes in sprinkle capsules that can be broken open and sprinkled onto soft food.
The exact way that Topamax works in the body isn’t fully understood. Topamax is thought to decrease abnormal excitement in the brain. Among other actions, Topamax affects the activity of the neurotransmitter gamma-aminobutyrate (GABA).
GABA is involved in the excitability of the nervous system. Problems with the GABA system has also been thought to play a role in the development of psychiatric disorders, including anxiety and depression.Cryan JF, et al. (2010). GABAB receptors and depression. Current status. DOI: 1016/S1054-3589(10)58016-5
There are many potential side effects of Topamax.
topamax side effects
- tingling of the arms and legs (paresthesia)
- not feeling hungry
- weight loss
- speech problems
- fatigue
- dizziness or sleepiness
- slow reactions (psychomotor slowing)
- nervousness
- abnormal vision
- fever
- difficulty with memory
- a change in the way foods taste (taste perversion)
- nausea
- diarrhea
- reduced sense of touch or sensation (hypoesthesia)
- stomach pain
- upper respiratory tract infection
These symptoms can be very serious:
- eye problems, including acute myopia (nearsightedness) and secondary closed angle glaucoma, visual field defects, and vision loss
- decreased sweating and increased body temperature (fever) metabolic acidosis (increased level of acid in your blood)
- suicidal thoughts
- kidney stones
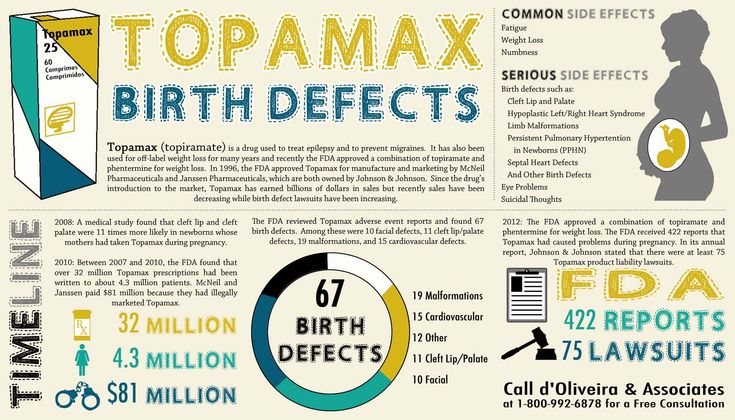
If you’re pregnant, you should speak to your doctor before taking Topamax. Topamax can cause harm to the fetus. Infants exposed to Topamax in utero have an increased risk of cleft lip, cleft palate, and low weight.
Topamax can cause harm to the fetus. Infants exposed to Topamax in utero have an increased risk of cleft lip, cleft palate, and low weight.
In 1996 the FDA approved Topamax for the treatment of partial onset or primary generalized tonic-clonic seizures and for people with seizures associated with Lennox-Gastaut syndrome.
Topiramate was also approved in 2012 for use in combination with another drug called phentermine for weight loss. This product goes by the brand name Qsymia.Vivus Inc. (2010). Vivus announces FDA approval of once daily qsymia (phentermine and topiramate extended-release) capsules CIV [Press release]. (2012). https://www.prnewswire.com/news-releases/vivus-announces-fda-approval-of-once-daily-qsymia-phentermine-and-topiramate-extended-release-capsules-civ-162810516.html
In 2014, the FDA approved Topamax for the prophylaxis (prevention) of migraine in patients 12 years of age and older.Janssen Pharmaceutical Inc. (2014). FDA OKs Janssen Pharmaceutical Inc. ‘s Topamax For Migraine Prevention In Adolescents [Press release]. https://www.biospace.com/article/releases/fda-oks-janssen-pharmaceutical-inc-s-topamax-for-migraine-prevention-in-adolescents-/
‘s Topamax For Migraine Prevention In Adolescents [Press release]. https://www.biospace.com/article/releases/fda-oks-janssen-pharmaceutical-inc-s-topamax-for-migraine-prevention-in-adolescents-/
The exact way that Topamax works to help prevent migraine isn’t known. One theory is that Topamax calms overactive nerve system cells in the brain that lead to migraine attacks.
Topamax is sometimes prescribed “off label” for other conditions. Off label means the drug is used to treat a condition for which it is not approved.
Prescribing a drug off label is not illegal, though it is illegal for a drug manufacturer to market the drug specifically for the off-label use. Your doctor will assess your symptoms and history to determine if he/she thinks that using Topamax off label will help you.
conditions treated by topamax
- seizures
- migraine
- obesity/weight loss
- PTSD
- bipolar disorder
- eating disorders, including binge eating disorder and bulimia
- alcohol addiction
- cocaine addiction
- painful nerve conditions
Topamax isn’t approved to treat depression or bipolar disorder with depression, but it may be helpful for people who have not found relief with other mood-stabilizing drugs. For this reason, a doctor may decide, after careful evaluation, to prescribe Topamax off label for treating depression.
For this reason, a doctor may decide, after careful evaluation, to prescribe Topamax off label for treating depression.
On the other hand, Topamax may also cause serious depression and suicidal thoughts in some people, so it’s important that you discuss this option carefully with your doctor.
If you’re thinking about using Topamax to treat depression, you should discuss whether or not the potential benefits outweigh the risks before making a decision.
If you’re already taking Topamax and you’re feeling depressed or you’re having thoughts of suicide or self-harm, call your doctor right away. Your doctor will help you figure out if need to adjust the dose or try a new medication instead.
How Topamax May Treat and Cause Depression
Topamax is the brand name for the drug topiramate. Topamax is approved to treat seizure disorders, like epilepsy, and to prevent migraine in adults.
Some people use Topamax to treat other conditions, like anxiety, depression, bipolar disorder, or post-traumatic stress disorder (PTSD), but Topamax is not approved by the Food and Drug Administration (FDA) for these purposes.
Though a few small studies have shown promise for using Topamax to treat depression or bipolar disorder with depression, there have been no large, peer-reviewed studies that show definitively that Topamax is safe and effective for these conditions.
In one small 2002 study of 16 women with treatment-resistant depression, 44 percent treated with Topamax reported improvement after 18 weeks.Carpenter L. (2002). Do obese depressed patients respond to topiramate? A retrospective chart review. https://www.ncbi.nlm.nih.gov/pubmed/12103474/
A more recent double-blind, placebo-controlled clinical trial was composed of 42 patients with major depressive disorder (MDD) who failed to respond to at least eight weeks of treatment with a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine, citalopram or sertraline.Mowla A, et al. (2011). Topiramate augmentation in patients with resistant major depressive disorder: A double-blind placebo-controlled clinical trial. DOI: 10.1016/j.pnpbp.2011.01.016
DOI: 10.1016/j.pnpbp.2011.01.016
The study found that the participants taking Topamax in addition to their prescribed depression medication significantly improved depressed mood, suicidality, insomnia, agitation, and anxiety symptoms compared to those taking a placebo.
In another randomized, single-blind study, individuals with bipolar disorder in the depressive phase showed a significant improvement of symptoms in 56 percent of patients treated with topiramate.McIntyre RS, et al. (2002). Topiramate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. https://www.ncbi.nlm.nih.gov/pubmed/12180276/
This was compared to 59 percent of patients who received another common anti-depressant known as bupropion (Wellbutrin). However, like the other studies mentioned above, this study was small, including a total of 36 patients.
Larger clinical trials will be needed to confirm Topamax’s use in treating depression or bipolar depression before the drug can be approved for this condition.
Still, some doctors may choose to prescribe Topamax off label. Your doctor may decide to do this if several other antidepressant or mood-stabilizing drugs fail to control your symptoms.
Since one of the side effects of Topamax is weight loss, a doctor may also decide to prescribe Topamax alongside another antidepressant as an adjunctive therapy to help offset any weight gain that may have been caused by the antidepressant.Mahmood S, et al. (2013). Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. DOI: 1097/JCP.0b013e31827cb2b7
There have been a few reports of Topamax causing or worsening depression in people taking it for other conditions, such as seizures, migraines, or bipolar disorder.Klufas A, et al. (2001). Letters to the editor: Topiramate-Induced Depression. https://ajp.psychiatryonline.org/doi/pdf/10.1176/appi.ajp.158.10.1736
Topamax may increase a person’s risk of experiencing suicidal thoughts or behavior (thinking about hurting or killing yourself). About 1 in every 500 people who took anticonvulsants like Topamax during clinical studies became suicidal.Topamax (topiramate) medication guide. (2018). http://www.janssenlabels.com/package-insert/product-patient-information/TOPAMAX-medication-guide.pdf
About 1 in every 500 people who took anticonvulsants like Topamax during clinical studies became suicidal.Topamax (topiramate) medication guide. (2018). http://www.janssenlabels.com/package-insert/product-patient-information/TOPAMAX-medication-guide.pdf
symptoms to report if you take topamax
- new depression or worsening of depression
- suicidal thoughts
- attempts to commit suicide
- new or worsening anxiety
- irritability
- trouble sleeping
- panic attacks
- an extreme increase in activity and talking (mania)
- withdrawing from friends and family
- unusual changes in mood or behavior
Topamax is a prescription drug that belongs to a class of drugs called anticonvulsants or antiepileptic drugs (AEDs). It’s described on its FDA label as a “sulfamate-substituted monosaccharide.”Topamax (topiramate) label. (2017). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020505s057_020844s048lbl. pdf
pdf
Topamax tablets come as 25 milligrams (mg), 50 mg, 100 mg, and 200 mg round tablets that are taken whole by mouth. The drug also comes in sprinkle capsules that can be broken open and sprinkled onto soft food.
The exact way that Topamax works in the body isn’t fully understood. Topamax is thought to decrease abnormal excitement in the brain. Among other actions, Topamax affects the activity of the neurotransmitter gamma-aminobutyrate (GABA).
GABA is involved in the excitability of the nervous system. Problems with the GABA system has also been thought to play a role in the development of psychiatric disorders, including anxiety and depression.Cryan JF, et al. (2010). GABAB receptors and depression. Current status. DOI: 1016/S1054-3589(10)58016-5
There are many potential side effects of Topamax.
topamax side effects
- tingling of the arms and legs (paresthesia)
- not feeling hungry
- weight loss
- speech problems
- fatigue
- dizziness or sleepiness
- slow reactions (psychomotor slowing)
- nervousness
- abnormal vision
- fever
- difficulty with memory
- a change in the way foods taste (taste perversion)
- nausea
- diarrhea
- reduced sense of touch or sensation (hypoesthesia)
- stomach pain
- upper respiratory tract infection
These symptoms can be very serious:
- eye problems, including acute myopia (nearsightedness) and secondary closed angle glaucoma, visual field defects, and vision loss
- decreased sweating and increased body temperature (fever) metabolic acidosis (increased level of acid in your blood)
- suicidal thoughts
- kidney stones
If you’re pregnant, you should speak to your doctor before taking Topamax. Topamax can cause harm to the fetus. Infants exposed to Topamax in utero have an increased risk of cleft lip, cleft palate, and low weight.
Topamax can cause harm to the fetus. Infants exposed to Topamax in utero have an increased risk of cleft lip, cleft palate, and low weight.
In 1996 the FDA approved Topamax for the treatment of partial onset or primary generalized tonic-clonic seizures and for people with seizures associated with Lennox-Gastaut syndrome.
Topiramate was also approved in 2012 for use in combination with another drug called phentermine for weight loss. This product goes by the brand name Qsymia.Vivus Inc. (2010). Vivus announces FDA approval of once daily qsymia (phentermine and topiramate extended-release) capsules CIV [Press release]. (2012). https://www.prnewswire.com/news-releases/vivus-announces-fda-approval-of-once-daily-qsymia-phentermine-and-topiramate-extended-release-capsules-civ-162810516.html
In 2014, the FDA approved Topamax for the prophylaxis (prevention) of migraine in patients 12 years of age and older.Janssen Pharmaceutical Inc. (2014). FDA OKs Janssen Pharmaceutical Inc. ‘s Topamax For Migraine Prevention In Adolescents [Press release]. https://www.biospace.com/article/releases/fda-oks-janssen-pharmaceutical-inc-s-topamax-for-migraine-prevention-in-adolescents-/
‘s Topamax For Migraine Prevention In Adolescents [Press release]. https://www.biospace.com/article/releases/fda-oks-janssen-pharmaceutical-inc-s-topamax-for-migraine-prevention-in-adolescents-/
The exact way that Topamax works to help prevent migraine isn’t known. One theory is that Topamax calms overactive nerve system cells in the brain that lead to migraine attacks.
Topamax is sometimes prescribed “off label” for other conditions. Off label means the drug is used to treat a condition for which it is not approved.
Prescribing a drug off label is not illegal, though it is illegal for a drug manufacturer to market the drug specifically for the off-label use. Your doctor will assess your symptoms and history to determine if he/she thinks that using Topamax off label will help you.
conditions treated by topamax
- seizures
- migraine
- obesity/weight loss
- PTSD
- bipolar disorder
- eating disorders, including binge eating disorder and bulimia
- alcohol addiction
- cocaine addiction
- painful nerve conditions
Topamax isn’t approved to treat depression or bipolar disorder with depression, but it may be helpful for people who have not found relief with other mood-stabilizing drugs. For this reason, a doctor may decide, after careful evaluation, to prescribe Topamax off label for treating depression.
For this reason, a doctor may decide, after careful evaluation, to prescribe Topamax off label for treating depression.
On the other hand, Topamax may also cause serious depression and suicidal thoughts in some people, so it’s important that you discuss this option carefully with your doctor.
If you’re thinking about using Topamax to treat depression, you should discuss whether or not the potential benefits outweigh the risks before making a decision.
If you’re already taking Topamax and you’re feeling depressed or you’re having thoughts of suicide or self-harm, call your doctor right away. Your doctor will help you figure out if need to adjust the dose or try a new medication instead.
what you need to know - Drink-Drink
Topamax is the brand name for topiramate. Topamax is approved for the treatment of seizure disorders such as epilepsy and for the prevention of migraine in adults.
Some people use Topamax to treat other conditions such as anxiety, depression, bipolar disorder, or post-traumatic stress disorder (PTSD), but Topamax is not approved by the Food and Drug Administration (FDA) for these uses.
Can Topamax help with depression?
Although several small studies have demonstrated the promise of using Topamax for the treatment of depression or bipolar disorder with depression, there have been no large peer-reviewed studies that definitively show that Topamax is safe and effective in these conditions.
In one small 2002 study of 16 women with treatment-resistant depression, 44 percent of those treated with Topamax reported improvement after 18 weeks. Carpenter L. (2002). Do obese depressed patients respond to topiramate? Retrospective review of the chart. https://www.ncbi.nlm.nih.gov/pubmed/12103474/
A more recent, double-blind, placebo-controlled clinical trial included 42 patients with major depressive disorder (MDD) who did not respond to at least eight weeks of treatment with a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine, citalopram, or sertraline. Maula A. et al. (2011). Dose escalation of topiramate in patients with resistant major depressive disorder: a double-blind, placebo-controlled clinical trial. DOI: 10.1016/j.pnpbp.2011.01.016
DOI: 10.1016/j.pnpbp.2011.01.016
A study showed that participants who took Topamax in addition to their prescribed depression medications significantly improved depressed mood, suicidality, insomnia, agitation, and anxiety symptoms compared to those who took a placebo.
In another randomized, single-blind study in individuals with bipolar disorder in the depressive phase, there was a significant improvement in symptoms in 56% of patients treated with topiramate. McIntyre R.S. et al. (2002). Topiramate versus bupropion SR when added to mood stabilizer therapy in the depressive phase of bipolar disorder: a preliminary single-blind study. https://www.ncbi.nlm.nih.gov/pubmed/12180276/
This compares with 59 percent of patients who received another common antidepressant known as bupropion (wellbutrin). However, like the other studies mentioned above, this study was small and included only 36 patients.
Larger clinical trials will be required to confirm the use of Topamax in the treatment of depression or bipolar depression before it is approved for this condition.
However, some physicians may prescribe Topamax off label. Your doctor may decide to do this if several other antidepressants or mood-stabilizing drugs are not working for your symptoms.
Because one of the side effects of Topamax is weight loss, your doctor may also decide to prescribe Topamax along with another antidepressant as an adjunctive therapy to help offset any weight gain that the antidepressant may have caused. Mahmoud S. et al. (2013). Effect of topiramate on weight gain in patients receiving atypical antipsychotics. DOI: 1097/JCP.0b013e31827cb2b7
Can Topamax cause depression?
There have been several reports of Topamax causing or worsening depression in people taking it for other conditions such as seizures, migraine or bipolar disorder. Klufas A. et al. (2001). Letters to the editor: Topiramate-induced depression. https://ajp.psychiatryonline.org/doi/pdf/10.1176/appi.ajp.158.10.1736
Topamax may increase a person's risk of suicidal thoughts or behavior (thoughts of harming themselves or committing suicide). Approximately 1 out of every 500 people who took anticonvulsants such as Topamax during clinical trials became suicidal. Topamax (topiramate) drug guide. (2018). http://www.janssenlabels.com/package-insert/product-patient-information/TOPAMAX-medication-guide.pdf
Approximately 1 out of every 500 people who took anticonvulsants such as Topamax during clinical trials became suicidal. Topamax (topiramate) drug guide. (2018). http://www.janssenlabels.com/package-insert/product-patient-information/TOPAMAX-medication-guide.pdf
Symptoms that should be reported if you take Topamax
- New Depression or exacerbation of depression
- Thoughts of suicide
- Suicide attempts
- New or increasing anxiety
- Problems of
excessive increase in activity and talking (mania)- withdrawal from friends and family
- unusual changes in mood or behavior
What is Topamax?
Topamax is a prescription drug that belongs to a class of drugs called anticonvulsants or antiepileptic drugs (AEDs). It is described on the FDA label as "monosaccharide substituted with sulfamate". Topamax (topiramate) label. (2017). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020505s057_020844s048lbl.pdf
(2017). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020505s057_020844s048lbl.pdf
Topamax tablets are available as round tablets of 25 milligrams (mg), 50 mg, 100 mg and 200 mg taken by mouth entirely. The drug is also available in sprinkle capsules that can be broken up and sprinkled on soft foods.
The exact action of Topamax in the body is not fully understood. Topamax is believed to reduce abnormal excitation in the brain. Among other things, Topamax affects the activity of the neurotransmitter gamma-aminobutyrate (GABA).
GABA is involved in the excitability of the nervous system. Problems with the GABA system are also believed to play a role in the development of psychiatric disorders, including anxiety and depression. Cryan J. F. et al. (2010). GABAB receptors and depression. Current status. DOI: 1016/S1054-3589(10)58016-5
What are the side effects of Topamax?
There are many potential side effects of Topamax.
Side effects Topamax
- Tingling in the hands and legs (paresthesia)
- I do not feel hunger
- Loss in weight
- Problems with speech
- Fatigence
- Swear
- nervousness
- Abnormal vision
- fever
- Difficulties with memory
- Changes in the taste of food (perversion of taste)
- Nausea
- DIOLS
- Reducing or sensitivity (Hypestesia)
- Lumber of belly
0 symptoms can be very severe:
- vision problems, including acute myopia (nearsightedness) and secondary angle-closure glaucoma, visual field defects and loss of vision
- decreased sweating and increased body temperature (fever) metabolic acidosis (increased acid levels in the blood)
- thoughts of suicide
- kidney stones
If you are pregnant, you should talk to your doctor before taking Topamax. Topamax may harm the fetus. Babies exposed to Topamax in utero have an increased risk of cleft lip, cleft palate, and low birth weight.
Topamax may harm the fetus. Babies exposed to Topamax in utero have an increased risk of cleft lip, cleft palate, and low birth weight.
What does Topamax treat? Why is it prescribed?
In 1996, the FDA approved Topamax for the treatment of partial or primary generalized tonic-clonic seizures and for people with seizures associated with Lennox-Gastaut syndrome.
In 2012, topiramate was also approved for use in combination with another drug called phentermine for weight loss. This product is branded as Qsymia. Vivus Inc. (2010). Vivus Announces FDA Approval of CIV qsymia (Phentermine and Topiramate Extended Release) Once Daily Capsules [Press Release]. (2012). https://www.prnewswire.com/news-releases/vivus-announces-fda-approval-of-once-daily-qsymia-phentermine-and-topiramate-extended-release-capsules-civ-162810516.html
In 2014, the FDA approved Topamax for the prevention (prevention) of migraine in patients 12 years of age and older. Janssen Pharmaceutical Inc. (2014). FDA approved Janssen Pharmaceutical Inc.'s Topamax. for the prevention of migraine in adolescents [press release]. https://www.biospace.com/article/releases/fda-oks-janssen-pharmaceutical-inc-s-topamax-for-migraine-prevention-in-adolescents-/
(2014). FDA approved Janssen Pharmaceutical Inc.'s Topamax. for the prevention of migraine in adolescents [press release]. https://www.biospace.com/article/releases/fda-oks-janssen-pharmaceutical-inc-s-topamax-for-migraine-prevention-in-adolescents-/
The exact way Topamax helps prevent migraines , unknown. One theory is that Topamax calms the overactive nervous system cells in the brain that lead to migraine attacks.
Topamax is sometimes prescribed off label for other conditions. Off label means the drug is being used to treat a condition for which it is not approved.
Off-label prescribing is not illegal, although the drug manufacturer may not sell the drug specifically for off-label use. Your doctor will evaluate your symptoms and history to determine if he/she thinks off-label use of Topamax will help you.
On the other hand, Topamax can also cause severe depression and suicidal thoughts in some people, so it is important that you carefully discuss this option with your doctor.
If you are considering using Topamax to treat depression, you should discuss whether the potential benefits outweigh the risks before making a decision.
If you are already taking Topamax and feel depressed or have thoughts of suicide or self-harm, call your doctor right away. Your doctor will help you figure out if you need to adjust your dose or try a new medication instead.
how Topamax can treat and cause depression
Topamax is the brand name for topiramate. Topamax is approved for the treatment of seizure disorders such as epilepsy and for the prevention of migraine in adults.
Topamax is used by some to treat other conditions such as anxiety, depression, bipolar disorder, or post-traumatic stress disorder (PTSD), but Topamax has not been approved by the Food and Drug Administration (FDA) for this purpose.
contents
Can Topamax help with depression?
Although several small studies have demonstrated the promise of using Topamax for the treatment of depression or bipolar disorder with depression, no large peer-reviewed study has been conducted that definitively shows that Topamax is safe and effective in these conditions.
In a small 2002 study of 16 women with treatment-resistant depression, 44 percent of patients treated with Topamax reported improvement after 18 weeks. Carpenter L. (2002). Do obese depressed patients respond to topiramate? Retrospective review of the graph. https://www.ncbi.nlm.nih.gov/pubmed/12103474/
A recent double-blind, placebo-controlled clinical trial involved 42 patients with major depressive disorder (MDD) who did not respond to at least eight weeks of treatment with a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine, citalopram, or sertraline. Mowla A et al. (2011). Topiramate elevation in patients with resistant major depressive disorder: a double-blind, placebo-controlled clinical trial. DOI: 10.1016 / j.pnpbp.2011.01.016
A study found that participants who took Topamax with their prescribed depression medication experienced significant improvements in depressed mood, suicide, insomnia, anxiety, and anxiety symptoms compared to those who took a placebo.
In another randomized, single-blind study in individuals with bipolar disorder in the depressive phase, there was a significant improvement in symptoms in 56% of patients treated with topiramate. McIntyre RS, et al. (2002). Topiramate versus bupropion SR when added to mood stabilizer therapy in the depressive phase of bipolar disorder: a preliminary single-blind study. https://www.ncbi.nlm.nih.gov/pubmed/12180276/
This compares with 59 percent of patients who received another common antidepressant known as bupropion (wellbutrin). However, like the other studies mentioned above, this study was small and included only 36 patients.
Evidence for the use of Topamax in the treatment of depression or bipolar depression will require larger clinical trials before the drug is approved for the treatment of this condition.
However, some doctors may decide to prescribe Topamax off-label. Your doctor may decide to do this if certain other antidepressants or mood stabilizers do not control your symptoms.
Because one of the side effects of Topamax is weight loss, your doctor may also decide to prescribe Topamax along with other antidepressants as an adjunctive therapy to help offset any weight gain that the antidepressant may have caused. Mahmoud S. et al. (2013). Effect of topiramate on weight gain in patients receiving atypical antipsychotics. DOI: 1097/JCP.0b013e31827cb2b7
Can Topamax cause depression?
There have been several reports of Topamax causing or worsening depression in people who take it for other conditions such as seizures, migraine or bipolar disorder. Klufas A. et al. (2001). Letters to the editor: Topiramate-induced depression. https://ajp.psychiatryonline.org/doi/pdf/10.1176/appi.ajp.158.10.1736
Topamax may increase a person's risk of suicidal thoughts or behavior (thoughts of being hurt or killed). Approximately 1 out of every 500 people who took anticonvulsants such as Topamax during clinical trials became suicidal. (2018). http://www. janssenlabels.com/package-insert/product-patient-information/TOPAMAX-medication-guide.pdf
janssenlabels.com/package-insert/product-patient-information/TOPAMAX-medication-guide.pdf
What is Topamax?
Topamax is a prescription drug that belongs to a class of drugs called anticonvulsants or antiepileptics (AEDs). It is described on the FDA label as a monosaccharide substituted with sulfamide. Sticker Topamax (topiramate). (2017). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020505s057_020844s048lbl.pdf
Topamax tablets are available as round tablets of 25 milligrams (mg), 50 mg, 100 mg, and 200 mg taken throughout all the way. The medicine is also contained in spray capsules that can be split and sprinkled on soft food.
The exact action of Topamax in the body is not fully understood. Topamax is believed to reduce abnormal excitation in the brain. Among other things, Topamax affects the activity of the neurotransmitter gamma-aminobutyrate (GABA).
GABA is involved in the excitability of the nervous system. Problems with the GABA system are believed to play a role in the development of psychiatric disorders, including anxiety and depression. Cryan J. F. et al. (2010). GABAB receptors and depression. Current status. DOI: 1016/S1054-3589(10)58016-5
Cryan J. F. et al. (2010). GABAB receptors and depression. Current status. DOI: 1016/S1054-3589(10)58016-5
What are the side effects of Topamax?
There are many potential side effects of Topamax.
Side effects Topamax
- Tingling in the hands and legs (paresthesia)
- I do not feel hunger
- Loss weight weight
- Problems with speech
- Humor
- Distribution or drowsiness
- Slow reaction (psychomotor minimum use)
- abnormal vision
- High temperature
- Difficulties with memory
- Changes in the taste of food (perversion of taste)
- Nausea
- Diariya
- Reducing or sensitivity (Hypoesthesia)
- pain in the abdomen
- The infection very serious:
- vision problems, including acute myopia (nearsightedness) and secondary angle-closure glaucoma, visual field impairment and loss of vision
- decreased sweating and fever (fever) metabolic acidosis (increased acid levels in the blood)
- suicidal thoughts
- kidney stones
If you are pregnant, you should consult your doctor before taking Topamax.
 Topamax may harm the fetus. Infants exposed to Topamax in utero have an increased risk of lip formation, cleft palate and low birth weight.
Topamax may harm the fetus. Infants exposed to Topamax in utero have an increased risk of lip formation, cleft palate and low birth weight. What does Topamax treat? Why is it prescribed?
In 1996, the FDA approved Topamax for the treatment of partial seizures or primary generalized tonic-clonic seizures and for patients with seizures associated with Lennox-Gastaut syndrome.
In 2012, topiramate was also approved for use in combination with another drug called phentermine for weight loss. This product is branded as Qsymia.Vivus Inc. (2010). Vivus Announces FDA Approval of qsymia Once Daily (Phentermine and Topiramate) CIV Capsules [Press Release]. (2012). https://www.prnewswire.com/news-releases/vivus-announces-fda-approval-of-once-daily-qsymia-phentermine-and-topiramate-extended-release-capsules-civ-162810516.html
In 2014, the FDA approved Topamax for the prevention of migraine in patients over 12 years of age. Janssen Pharmaceutical Inc. (2014). FDA approved Janssen Pharmaceutical Inc.
 with Topamax for migraine prevention in adolescents [press release]. https://www.biospace.com/article/releases/fda-oks-janssen-pharmaceutical-inc-s-topamax-for-migraine-prevention-in-adolescents-/
with Topamax for migraine prevention in adolescents [press release]. https://www.biospace.com/article/releases/fda-oks-janssen-pharmaceutical-inc-s-topamax-for-migraine-prevention-in-adolescents-/ The exact mode of action of Topamax to prevent migraine is unknown. One theory is that Topamax calms overactive nervous system cells in the brain, which leads to migraine attacks.
Topamax is sometimes prescribed off label for other conditions. Off label indicates that a drug is being used to treat a condition for which it is not approved.
Off-label prescribing is not illegal, although it is illegal for a drug manufacturer to sell a drug specifically for off-label use. Your doctor will evaluate your symptoms and history to determine if off-label use of Topamax will help you.
Essence
Topamax is not approved for the treatment of depression or bipolar disorder with depression, but may be useful for people who have not been helped by other mood-stabilizing drugs.

Learn more
/nginx/o/2019/08/15/12467881t1he799.jpg)