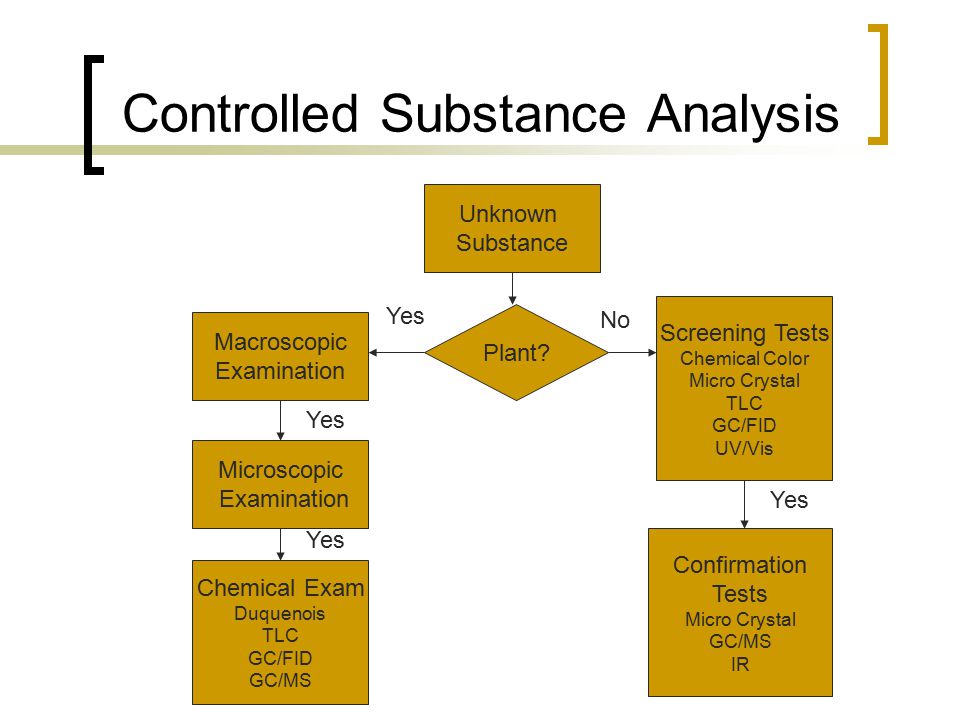
Buspirone a controlled substance
Is BuSpar (Buspirone) A Controlled Substance?
BuSpar is an antianxiety medication that is not considered a controlled substance and may be a safer alternative to Xanax for some people. However, BuSpar can still be dangerous in high doses, especially when mixed with other substances like alcohol.
BuSpar is a prescription medication that is often used to treat generalized anxiety disorder and panic disorder.
BuSpar has been discontinued as a brand name because there are so many generic versions of buspirone hydrochloride (buspirone HCL) currently available.
Buspirone has been found to be effective in the short-term treatment of anxiety and symptoms of anxiety.
It is often prescribed to a person in addition to certain antidepressants as a way of assisting them through especially stressful periods of time.
While BuSpar shares similarities with drugs like Xanax (alprazolam) and Valium (diazepam) in what it treats and potential side effects, it is not considered a narcotic or controlled substance.
Why BuSpar Is Not Considered A Controlled Substance
BuSpar is not a controlled substance because it is generally considered safe and non-addictive, unlike other drugs which have the same effects.
The biggest dangers with buspirone involve drug interactions.
Non-Habit-Forming
There is no current evidence to suggest that BuSpar is addictive or that people taking it will build up a tolerance to it.
People may, however, experience mild withdrawal symptoms when going off of it, which signals a buspirone dependence.
Less Sedative Properties Than Drugs Like Xanax
The effects of BuSpar, in general ,are similar to Xanax but more mild, including the sedative effects of the drug.
A high dose of buspirone will increase these sedative effects, but there has never been a recorded instance of someone overdosing on buspirone alone.
Works On Serotonin Receptors
Buspirone is considered safer than Xanax because it works on serotonin receptors as opposed to gamma-amino butyric acid (GABA) receptors.
GABA is a type of key neurotransmitter in the brain that impairs motor neurons and thus neuronal activity.
When these neurotransmitters are damaged over time with extended buspirone Xanax use, it can be detrimental to a person’s brain health.
What Drug Class Is Buspirone?
Buspirone is considered a non-benzodiazepine antianxiety agent. It can be referred to as an anxiolytic, or, a drug that is prescribed for treating anxiety.
Buspirone is usually not prescribed to someone as a first attempt at treating anxiety, and is more often prescribed only after someone has experienced adverse effects from a drug like Xanax.
How Buspar Works In The Body
While BuSpar works mostly by acting on serotonin neurotransmitters in the brain, there is also evidence to suggest that it works on dopamine neurotransmitters as well.
Serotonin and dopamine are both neurotransmitters that carry responsibility for a person’s mood and overall levels of happiness and pleasure.
One of the possible more serious side effects of buspirone is the development of a condition called serotonin syndrome.
This occurs when too much serotonin builds up in a person’s body and can be life-threatening if left untreated.
How Does Buspar Make You Feel?
As BuSpar is designed to treat anxiety disorders, it tends to make a person feel relaxed in order to combat their anxiety.
It can also make a person feel sleepy and tired, and even a little euphoric in some cases.
Common side effects of BuSpar include:
- nausea
- drowsiness
- headaches
- impairment
- lightheadedness
- slurred speech
- confusion
- chest pain
- weight gain
People may also experience withdrawal symptoms when going off BuSpar, but these are generally mild and can be avoided by tapering off the drug per healthcare provider instructions.
Is Buspar Dangerous?
BuSpar is not dangerous when taken as directed for a prescription drug.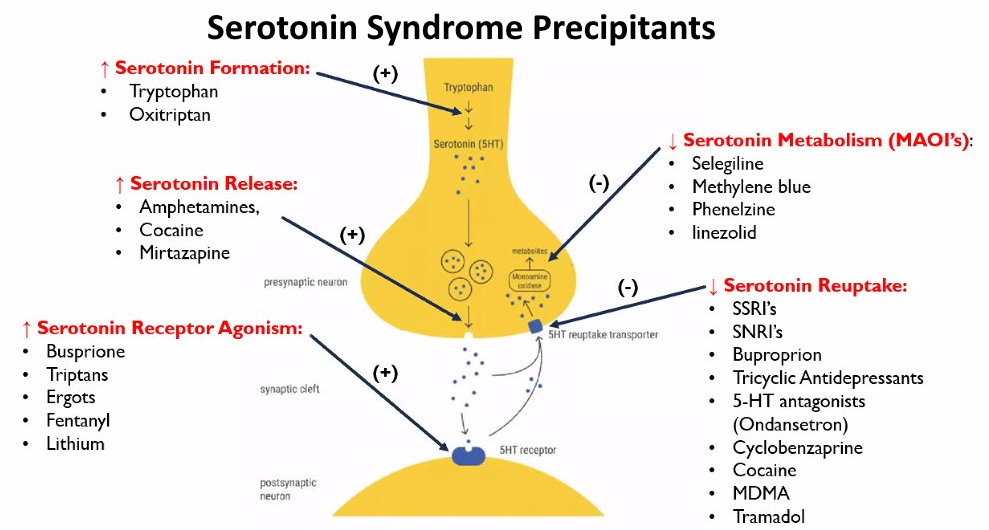 However, BuSpar can become dangerous when taken in high doses or when mixed with other substances, such as alcohol.
However, BuSpar can become dangerous when taken in high doses or when mixed with other substances, such as alcohol.
Grapefruit juice should be avoided while taking buspirone. Breastfeeding mothers should also avoid taking buspirone.
Buspirone can raise a person’s blood pressure to dangerous levels when combined with a type of drug known as monoamine oxidase inhibitors (MAOIs).
Drugs that may have potential interactions with buspirone include:
- erythromycin (a type of antibiotic)
- diltiazem (used for treating angina)
- haloperidol (used for treating mood disorders)
- itraconazole (an antifungal medication)
- nefazodone (a type of antidepressant)
- rifampin (a type of antibiotic)
- fluoxetine (a type of antidepressant)
- phenelzine (a type of antidepressant)
It is always important to be aware of any dangerous drug interactions and to follow the medical advice and directions of a healthcare professional.
Find Substance Abuse Treatment Services Today
BuSpar may not be considered dangerous on its own, but when abused with other drugs or substances, or taken in high doses, it comes with risks.
To discuss treatment options for yourself or a loved one, get in touch with us through our helpline today.
Written by the Addiction Resource Editorial Staff
This page does not provide medical advice. See more
Article resourcesAddiction Resource aims to provide only the most current, accurate information in regards to addiction and addiction treatment, which means we only reference the most credible sources available.
These include peer-reviewed journals, government entities and academic institutions, and leaders in addiction healthcare and advocacy. Learn more about how we safeguard our content by viewing our editorial policy.
- National Library of Medicine: MedlinePlus
https://medlineplus.gov/druginfo/meds/a688005.html - United States Drug Enforcement Administration
https://www.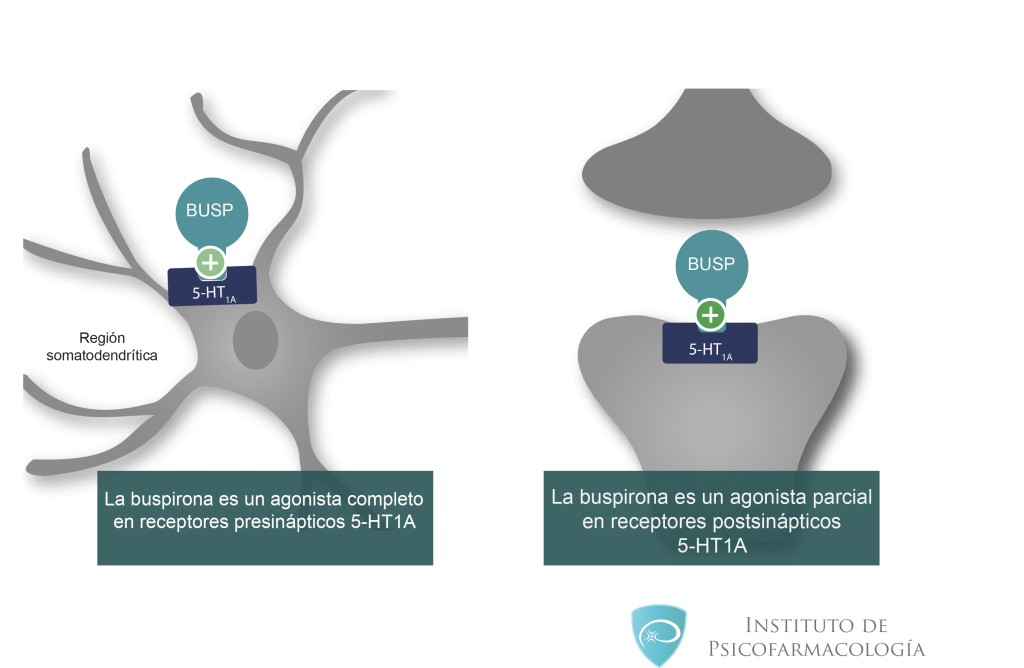 dea.gov/drug-information/drug-scheduling
dea.gov/drug-information/drug-scheduling - United States Food and Drug Administration (FDA)
https://www.accessdata.fda.gov/drugsatfda_docs/label/2001/18731s39s45lbl.pdf
- Was this Helpful?
- YesNo
BuSpar (Buspirone) Drug Class | What Type Of Drug Is Buspar?
- Is BuSpar A Controlled Substance?
- What Type Of Drug Is BuSpar?
Buspirone (brand name BuSpar) is an anxiolytic and anti-anxiety prescription drug used to help treat symptoms of anxiety for those suffering from certain anxiety disorders such as generalized anxiety disorder (GAD).
Is BuSpar A Controlled Substance?According to the United States Drug Enforcement Administration (DEA), BuSpar is not considered a controlled substance. This means the drug is unlikely to be abused or lead to addiction.
What Type Of Drug Is BuSpar?BuSpar is an anxiolytic medication, which means it’s an anti-anxiety drug.
However, the National Alliance on Mental Illness (NAMI) states BuSpar is unrelated to other anxiety medications and antidepressants such as benzodiazepines or barbiturates.
BuSpar binds to dopamine receptors in the brain and can affect the neurotransmitters in the brain providing relief for those suffering from anxiety.
Side Effects Of BuSparAlthough BuSpar may not be considered a controlled substance, it has common side effects.
Common Side EffectsAccording to the United States Food and Drug Administration (FDA), some of the common short-term side effects of buspirone may consist of:
- dizziness
- sedation
- lightheadedness
- drowsiness
- nausea
- dry mouth
- headache
- nervousness
Rare side effects may include:
- tardive dyskinesia (involuntary movements)
- weight fluctuations
- changes in blood pressure
- fainting
- serotonin syndrome which may cause hallucinations or seizures
A number of adverse effects can occur in those who take BuSpar, including allergic reactions and worsened medical conditions.
An allergic reaction may take place in those with a hypersensitivity to buspirone hydrochloride. Symptoms of an allergic reaction may lead to serious side effects such as chest pain, blurred vision, and an irregular heartbeat.
Those with certain medical conditions, including pregnancy, should seek the medical advice of their healthcare provider before taking BuSpar. Women who are breastfeeding should also speak with their doctor to determine the risk to the child if the drug can be passed through breast milk.
Patients with renal impairment should also avoid taking this prescription medication.
BuSpar Drug InteractionsVarious drug interactions may take place if BuSpar is combined with any of the substances or medications listed below:
- itraconazole (Onmel, Sporanox)
- certain vitamins or supplements
- diltiazem (Tiazasc, Dilacor, Cardizem)
- benzodiazepines such as alprazolam (Xanax), diazepam (Valium), and lorazepam (Ativan)
- haloperidol (Haldol)
- sleeping pills
- muscle relaxants
- nefazodone (Serzone)
- grapefruit juices
- erythromycin (E-Mycin, Erythrocin)
- alcohol
- verapamil (Covera, Verelan, Calan)
- opioids
- rifampin (Rifadin, Rimactane)
- anticonvulsants such as carbamazepine (Tegretol) and phenytoin (Phenytek, Dilantin)
- sedatives
- serotonin–norepinephrine reuptake inhibitors (SNRIs) such as venlafaxine (Effexor)
- barbiturates
- trazodone (Desyrel)
Those taking BuSpar should also avoid taking MAO inhibitors.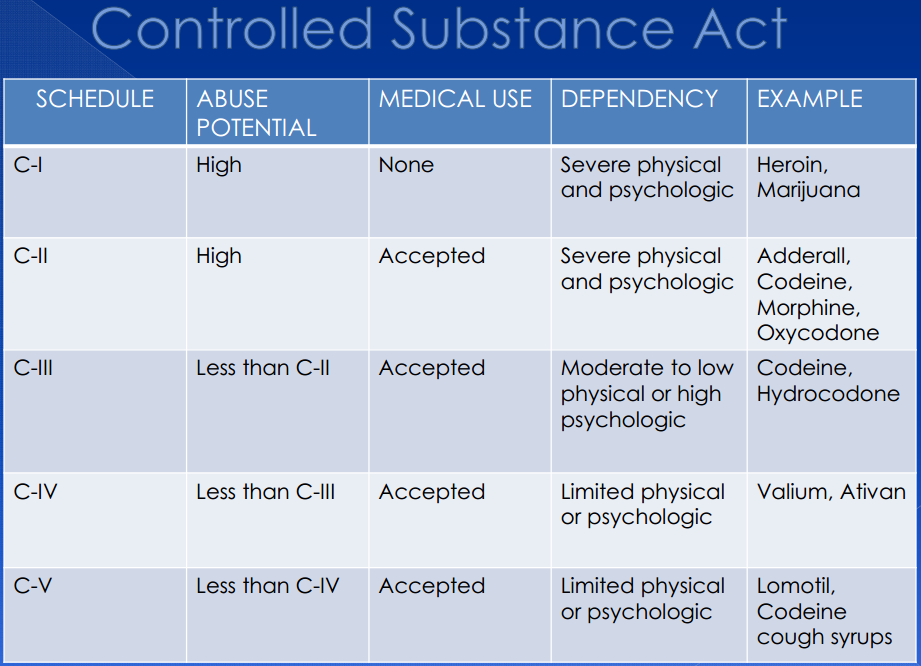 Examples of these inhibitors include:
Examples of these inhibitors include:
- phenelzine (Nardil)
- selegiline (Emsam, Zelapar, Eldepryl)
- methylene blue
- tranylcypromine (Parnate)
The use of buspirone with SSRIs can lead to serious mental health concerns. SSRIs include:
- fluoxetine (Sarafem, Prozac, Selfemra)
- fluvoxamine (Luvox)
- paroxetine (Pexeva, Brisdelle, Paxil)
- sertraline (Zoloft)
Serotonin syndrome may occur during long-term use. Withdrawal symptoms can occur if the prescription drug is stopped abruptly when combined with other medications, causing a person to experience irritability, insomnia, and tremors.
If you or a loved one struggles with prescription drug use, contact us today for information on our treatment options.
Written by Ark Behavioral Health Editorial Team
©2022 Ark National Holdings, LLC. | All Rights Reserved.
This page does not provide medical advice.

Sources
Food and Drug Administration - BuSpar
National Alliance on Mental Illness - Buspirone (BuSpar)
National Library of Medicine: MedlinePlus - Buspirone
National Library of Medicine: StatPearls - Buspirone
Ark Behavioral Health offers 100% confidential substance abuse assessment and treatment placement tailored to your individual needs. Achieve long-term recovery.
Call Now
100% confidential. We respect your privacy.
Prefer Texting?We've got you covered.
Receive 24/7 text support right away.
There is no obligation and you can opt out at any time.
First Name*
Phone Number*
By submitting this form you agree to the terms of use and privacy policy of the website. Message and data rates may apply. You may receive recourring messages. Text STOP to cancel.
| Rec. Included in preparations: list Pharmacological actionAnxiolytic agent, azaspirodecanedione derivative. The mechanism of action has not been definitively established. According to pharmacological properties, it does not apply to benzodiazepines or barbiturates. It has a selective anxiolytic effect. It does not have anticonvulsant activity, does not have a muscle relaxant effect. It does not have a pronounced sedative effect, especially at low doses. High affinity for serotonin 5-HT 1A receptors in dorsal raphe neurons and moderate affinity for dopamine D 2 receptors in the brain. Buspirone does not have a pronounced affinity for benzodiazepine receptors, it does not affect the binding of GABA. Unlike benzodiazepines, the level of spontaneous excitation of noradrenergic cells in the bluish locus (locus ceruleus) increases rather than decreases under the action of buspirone. Buspirone is not believed to cause tolerance, physical or psychological dependence. Pharmacokinetics After oral administration, it is rapidly absorbed from the gastrointestinal tract, Cmax max in blood plasma is reached after 40-90 minutes. Bioavailability is low due to the pronounced effect during the "first pass" through the liver. When taken with food, it is possible to increase bioavailability due to impaired absorption of buspirone from the gastrointestinal tract and, therefore, a decrease in presystemic clearance. Plasma protein binding of buspirone is about 95%. It undergoes intensive metabolism in the liver with the participation of the CYP3A4 isoenzyme. As a result of hydroxylation, several inactive metabolites are formed, oxidative dealkylation leads to the formation of 1-(2-pyrimidinyl)-piperazine, which has anxiolytic activity. Indications of the active substance BUSPIRONTreatment of anxiety disorders or temporary relief of symptoms of anxiety. Open list of ICD-10 codes
Dosage regimen Inside adults at the beginning of treatment - 5 mg 3 times / day. If necessary, the dose is increased by 5 mg / day every 2-3 days until the desired effect is achieved. Side effectsPossible: dizziness, headache, restlessness syndrome. Rare: blurred vision, decreased ability to concentrate, dry mouth, myalgia, muscle spasms, muscle cramps and rigidity, tinnitus, sleep disturbance, nightmares, general weakness, dyspepsia, chest pain, depression , tachycardia, paresthesia, sore throat, fever. Contraindications for useSevere renal impairment, severe hepatic impairment, hypersensitivity to buspirone. Pregnancy and lactationBuspirone should be avoided during pregnancy and lactation. Use in hepatic impairmentContraindicated in severe hepatic impairment. Use in impaired renal functionContraindicated in severe renal impairment. Use in childrenThe safety and efficacy of buspirone in children and adolescents under 18 years of age have not been established. Precautions Buspirone is not used to treat anxiety and reduce tension associated with the stresses of everyday life. Should not be used in patients with epilepsy or a history of a tendency to develop seizures. Use with caution in patients with hepatic and/or renal impairment. Patients with drug abuse or a history of drug dependence should be closely monitored during treatment to detect the development of buspirone tolerance or dependence. Controlled studies have shown that buspirone is not effective for long-term (more than 3-4 weeks) treatment of anxiety. However, when used for several months, no side effects were detected. If buspirone is used for a long time, then its effectiveness should be checked at regular intervals. During the period of treatment, the patient should refrain from activities associated with the need for concentration and high speed of psychomotor reactions. The safety and efficacy of buspirone in children and adolescents under 18 years of age have not been established. Drug interactions Buspirone is metabolized in the liver with the participation of the CYP3A4 isoenzyme and, apparently, can interact with drugs that are inhibitors or inducers of this isoenzyme. |
Active substance BUSPIRONUM | Compendium - drug reference book
- Pharmacological properties
- Indications BUSPIRONE
- Application of BUSPIRON
- Contraindications
- Side effects
- Special instructions
- Interactions
- Overdose
- Diagnosis
- Recommended alternatives
- Trading names
Medicines containing active substance Buspyron
Prices in pharmacies
Price in pharmacies
Spitomin ®
MG Blister, No. 60
9000tablets 10 mg blister, no. 60
Egis
Pharmacy prices
anxiolytic agent. Eliminates mental and vegetative symptoms of fear. The mechanism of action has not been fully elucidated, but buspirone is known to act differently than benzodiazepines and other anxiolytics. Shows a pronounced affinity for 5HT 9 serotonin receptors0016 1A and moderate - to dopamine D2 receptors in the brain. It does not have an anticonvulsant and muscle relaxant effect, it is not addictive. After stopping the use of buspirone, there is no withdrawal.
Shows a pronounced affinity for 5HT 9 serotonin receptors0016 1A and moderate - to dopamine D2 receptors in the brain. It does not have an anticonvulsant and muscle relaxant effect, it is not addictive. After stopping the use of buspirone, there is no withdrawal.
anxiety states of various origins, especially neuroses, accompanied by a feeling of anxiety, danger, restlessness, tension, worsening of sleep, irritability, as well as somatic disorders.
at the beginning of treatment, 5 mg is administered orally 3 times a day. To achieve the maximum therapeutic effect, the daily dose is increased by 5 mg with an interval of 2-3 days; the optimal daily dose is usually 20–30 mg; maximum - 45 mg.
hypersensitivity to buspirone, severe liver disease, impaired renal function, acute-angle glaucoma, myasthenia gravis, epilepsy, lactation period, age under 18 years.
dizziness, nausea, dry mouth, headache, tachycardia, irritability, agitation or drowsiness, fatigue. Buspirone does not cause drug dependence, however, if it is suddenly canceled, the side effects described above may develop.
Buspirone does not cause drug dependence, however, if it is suddenly canceled, the side effects described above may develop.
Buspirone is not an antipsychotic.
Does not prevent the development of withdrawal symptoms associated with long-term use of benzodiazepines; before starting treatment with buspirone, the use of anxiolytic drugs should be gradually discontinued.
Buspirone affects the psychophysical abilities of the body, reducing concentration and reaction time, especially when drinking alcohol or taking drugs that depress the central nervous system.
Do not prescribe buspirone during pregnancy if there are no absolute indications for its use. During treatment with buspirone, breastfeeding should be interrupted.
should not be administered simultaneously with MAO inhibitors due to an increased risk of developing hypertension. Buspirone increases the concentration of haloperidol in the blood serum. When combined with trazodone, the level of ALT increases by 3-6 times.
 INN WHO registered
INN WHO registered  These specific differences in the site of action are responsible for differences in the development of dependence and tolerance between benzodiazepines and buspirone.
These specific differences in the site of action are responsible for differences in the development of dependence and tolerance between benzodiazepines and buspirone.  T 1/2 buspirone is usually 2-4 hours, but the final T 1/2 can be 11 hours. It is excreted mainly as metabolites in the urine and also in the feces.
T 1/2 buspirone is usually 2-4 hours, but the final T 1/2 can be 11 hours. It is excreted mainly as metabolites in the urine and also in the feces. 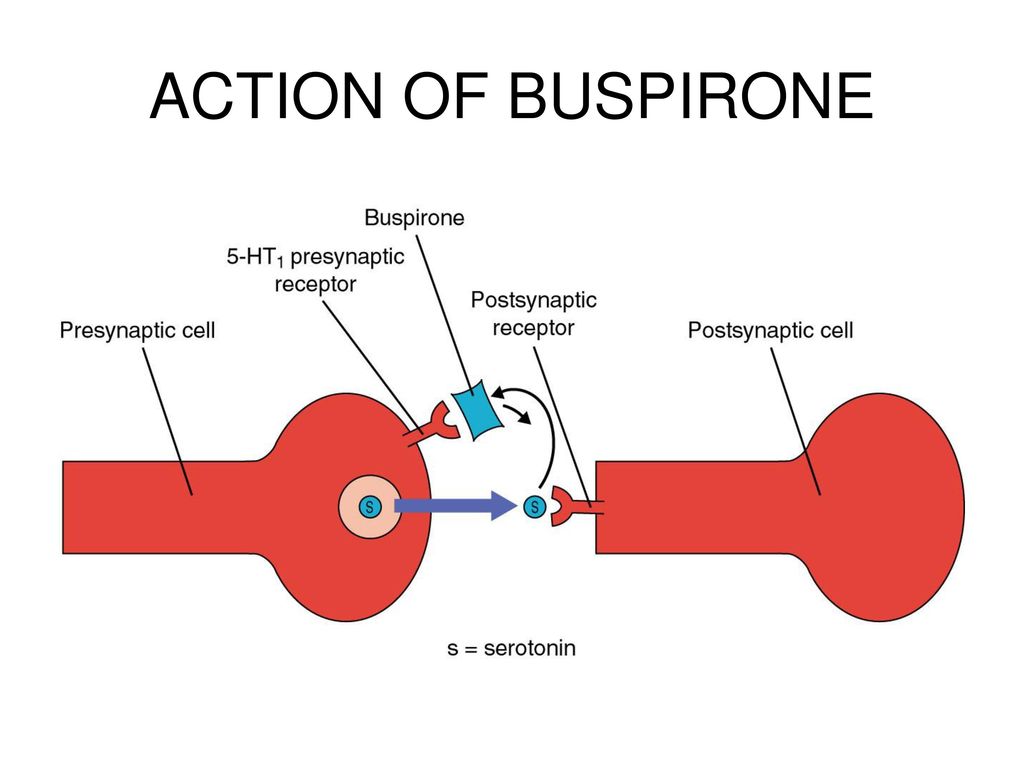 The maximum dose is 45-60 mg / day.
The maximum dose is 45-60 mg / day.