Abilify 2 mg weight gain
Abilify and Weight Gain: Causes & Contributing Factors
Abilify (Aripiprazole) is an atypical antipsychotic drug that is commonly used to treat severe psychiatric conditions like schizophrenia and bipolar disorder. In recent years, it has been heavily marketed as an antidepressant augmentation strategy. Despite the fact that this has been one of the most profitable medications of recent years, some research has suggested that its efficacy appears to be average.
In fact, most people end up eventually discontinuing treatment as a result of either the drug failing to treat their symptoms or tolerability issues. Like all antipsychotics, Abilify is capable of causing severe health problems such as neuroleptic malignant syndrome, high blood sugar, and increased risk of death. Additionally, even among individuals for which the drug is working well to manage symptoms, side effects like weight gain may be highly discouraging.
There is a lot of conflicting information regarding whether Abilify actually causes weight gain. Some doctors will explain that the drug is “weight neutral” and unlikely to affect your body-weight, while others may suggest that you’ll gain a marginal amount of weight throughout treatment. While compared to other atypical antipsychotics, Abilify tends to result in less “weight gain,” it doesn’t mean that the drug should be considered weight neutral for everyone.
The truth is that most people end up gaining some weight throughout their treatment with this drug. It is believed that its antagonist effect upon the 5-HT2C receptor is what may cause the gain.
How Abilify Causes Weight Gain
There are many ways in which this drug is capable of causing you to gain weight. Understand that what causes you to gain weight from this drug may be different for someone else. One person may experience an increase in appetite and start eating more, while another may believe their metabolism slowed. In other cases, a combination of these factors could lead to weight gain.
- Appetite increase: Some people that take Abilify notice that it makes them have a bigger appetite than they did prior to taking it.
 If you constantly feel hungry and find yourself eating whatever food is in sight, it’s probably related to the medication. For some individuals the increase in appetite is modest, while among others it’s significant.
If you constantly feel hungry and find yourself eating whatever food is in sight, it’s probably related to the medication. For some individuals the increase in appetite is modest, while among others it’s significant. - Blood sugar spikes: This is a drug that has been noted to affect levels of blood-glucose or blood sugars. When blood sugar levels are not stabilized, their instability may foster weight gain in some people. It is best to keep blood sugars in check throughout treatment and know that Abilify may be contributing to glucose spikes.
- Cravings for food: If you find yourself craving food throughout the day, it could be from this medication. Many people taking antipsychotics have noticed that they start to crave unhealthy foods such as refined carbohydrates and sugars (e.g. candy) during treatment. Craving food as a result of Abilify is a factor that may lead to weight gain.
- Fat storage: It is believed that antipsychotics have potential to modify the body’s fat storage mechanisms.
 This leads to more fat being stored in unwanted areas of the body and thus more overall weight gain. If your fat mass has significantly increased throughout treatment and your weight has gone up, it’s likely a result of Abilify.
This leads to more fat being stored in unwanted areas of the body and thus more overall weight gain. If your fat mass has significantly increased throughout treatment and your weight has gone up, it’s likely a result of Abilify. - Going out to eat: Assuming this drug helps with your psychiatric condition, you may start to feel more comfortable in public and/or may even start hanging out with friends again. Being social and more comfortable with yourself could lead you to go out to eat more frequently. Dining out tends to involve eating unhealthy foods with large portions. Additionally, if you start going to get “fast food,” it should be relatively obvious why you’re gaining weight.
- Hormone levels: Abilify is capable of altering levels of naturally occurring hormones throughout the body. Disrupting homeostatic hormone levels with an antipsychotic drug may account for weight gain in some cases. Any drug that significantly affects the hormones naturally produced by your body could result in substantial weight gain.

- Motivation deficit: Although the drug may affect everyone differently in terms of motivation, some people notice that their level of motivation significantly wanes throughout treatment. If you become less motivated to stay in shape or eat healthy, this makes it relatively easy to gain weight. Some people end up becoming lazy largely due to the fact that the drug has an uncontrollable influence over their physiology.
- Side effects: You may experience unwanted side effects from the drug such as fatigue, drowsiness, and sleepiness that make it difficult to get adequate physical exercise. These side effects may be so severe, that it becomes a challenge to get out of bed in the morning. If you are debilitated by fatigue, this may further slow your metabolism because you aren’t getting much physical activity to keep it high.
- Slow metabolism: If you are eating the same foods / portion sizes and getting the same amount of exercise during treatment as you were before taking Abilify, but are still gaining weight, it’s likely due to metabolic slowing.
 Drugs like Abilify have a tendency to slow your metabolism, resulting in weight gain even when your dietary and exercise habits haven’t changed. This can be frustrating, but is generally accepted as part of treatment.
Drugs like Abilify have a tendency to slow your metabolism, resulting in weight gain even when your dietary and exercise habits haven’t changed. This can be frustrating, but is generally accepted as part of treatment. - Taste improvement: People who were really depressed before taking Abilify may notice that food tastes way better during treatment. Food may start to taste so good, that you may feel as though you can’t get enough. The taste improvement is likely to lead to you consuming bigger portions, more calories, and inevitable weight gain.
Note: The severity to which these factors are experienced is subject to individual variation.
Factors that influence weight gain on Abilify
There are many other factors that may influence the amount of weight you gain on Abilify. It is important to consider the dosage you’re taking, personal lifestyle factors, the duration for which you’ve been medicated, as well as if you are taking other medications.
1. Dosage
Most people taking Abilify for schizophrenia or bipolar disorder end up taking between 10 mg and 15 mg daily, but dosage can be increased all the way up to 30 mg. In general, you are more likely to experience weight gain as you increase the dosage. This is due to the fact that at higher doses, the drug has more influence over your natural physiology.
Certain physiological functions such as the body’s ability to store fat, along with important hormone levels that help prevent weight gain, become drastically altered. At lower doses, a person is less likely to gain weight simply because the drug has less influence over your physiology. For this reason, it is always recommended to use a “minimal effective dose” strategy if you are concerned about weight gain.
2. Individual factors
In some cases, it is necessary to take personal responsibility for some of the weight that you gain throughout treatment. If you have been overworking, not getting enough sleep, are eating a poor diet, or aren’t getting much exercise, those are all factors that can contribute to weight gain. It is often important to consider that there are certain behaviors that you are exhibiting that are directly contributing to weight gain.
It is often important to consider that there are certain behaviors that you are exhibiting that are directly contributing to weight gain.
There are also other individual factors such as: age, baseline metabolism, and genetics that could influence whether you gain weight while taking this drug. In order to get a better understanding of how your genetics may be reacting to the drug (to cause weight gain), you could look into a test called “GeneSight.” This type of test analyzes some genetic biomarkers and predicts whether you will react favorably vs. unfavorably to a particular psychiatric medication.
3. Time span
The duration over which you have taken the drug may have an impact on the amount of weight you gain. Some people notice significant weight gain in the short-term (i.e. 6 weeks), while others notice more significant weight gain over the long-term (i.e. years). In any regard, it is thought that the longer duration over which you have been treated with this drug, the more likely you will have gained weight.
Short-term: The shorter the term over which you’ve been taking the drug, the less likely you will be to have gained weight. It should be noted that some people experience a major spike in weight during the early stages of treatment, with no further significant weight gain over the long-term. Others experience an initial spike in weight, followed by more gradual increases in weight over the long-term. There are cases of patients gaining 20 lbs. within just 60 days of treatment.
Long-term: Those that have been taking Abilify for a long period of time are more likely to have experienced some sort of weight gain compared to those who only took it for a short duration. Being treated over the long-term generally results in a greater severity of drug-induced physiological alterations. Therefore homeostatic functions and hormone levels will be further from the baseline. It should also be noted that as a person stays medicated over the long-term, they may develop a tolerance. This generally results in a dosage increase, which is known to promote further weight gain.
- Source: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3000187/
4. Other medications
There are studies suggesting that certain medications are capable of causing increased weight gain while on Abilify. If you are taking a serotonergic antidepressant (SSRI) along with Abilify, there is greater likelihood that you’ll gain more weight than someone taking only Abilify. If you are on multiple medications, it is important to review them with your doctor to determine which may be the primary culprit for your weight gain.
Also understand that if you are taking a stimulatory drug such as Adderall or a non-serotonergic antidepressant, you may remain weight neutral while taking Abilify. Those that remain weight neutral are generally on a medication to stimulate the central nervous system, which helps offset the metabolic slowing that stems from antipsychotic usage.
How much weight will you gain from Abilify?
There’s no predicting exactly how much weight you’re going to gain from taking Abilify. Since there is significant individual variation, you’ll need to first take the medication for awhile and then evaluate whether you’ve gained any weight. Some people have reported that they gained an average of 5 lbs. to 10 lbs. per year of treatment, while other people have gained 40 lbs. within the first 6-months of treatment.
Since there is significant individual variation, you’ll need to first take the medication for awhile and then evaluate whether you’ve gained any weight. Some people have reported that they gained an average of 5 lbs. to 10 lbs. per year of treatment, while other people have gained 40 lbs. within the first 6-months of treatment.
In other cases, people end up not gaining any weight. Although the average weight gain for adults taking Abilify is unknown, the experienced doctors know that on average, patients are probably going to gain at least 10% of their pre-drug body-weight as a result of the medication. Assuming you are 200 lbs. starting treatment, you may end up gaining at least 20 lbs.
Will everyone gain weight while taking Abilify?
Despite the fact that an overwhelming majority of people who take Abilify will gain weight, it doesn’t meant that everyone will experience noticeable weight gain. Some individuals may remain weight neutral (and in rare cases lose weight). Those that remain weight neutral generally are able to maintain a healthy diet, physical exercise, and/or are taking a medication that helps offset the weight-gaining effects of Abilify.
Those that remain weight neutral generally are able to maintain a healthy diet, physical exercise, and/or are taking a medication that helps offset the weight-gaining effects of Abilify.
It is also important to point out that for people in need of an antipsychotic, this is considered one of the most “weight neutral” options. Nearly all antipsychotics are likely to cause some sort of weight gain, but Abilify is suggested to cause less weight gain than most others. Some people may experience weight loss if they switched to Abilify from another antipsychotic (e.g. Zyprexa) that is known to cause greater weight gain.
“Weighing” the Pros and Cons of Abilify
Throughout your treatment, it is important for you to conduct a cost-benefit analysis of this drug. In other words, “weigh” the side effects (including weight gain) and determine their severity. Next take a look at how well the drug is working to treat or manage your condition. If Abilify is working great to treat your condition, but you experience some minor side effects, it’s probably a good idea to continue treatment.
On the other hand, if you have gained a bunch of weight and the drug just isn’t working very well, you should talk to your doctor. Generally if a drug isn’t working to treat your condition and/or side effects are interfering with your well-being, you may want to consider a medication switch or Abilify withdrawal. No medication is worth staying on if you aren’t getting benefit.
Did you gain weight while taking Abilify?
For those that took Abilify, be sure to share whether you experienced weight gain. Was it consistent with the idea that you’ll gain at least 10% body-weight throughout treatment? If you gained weight, be sure to mention how much weight you gained, whether you noticed you gained more weight when your dosage increased, and how long you’ve been taking Abilify. Also be sure to note any other possible factors that may have caused you to gain weight throughout your treatment.
- Source: http://www.ncbi.nlm.nih.gov/pubmed/23469329
Abilify Side Effects | Compulsive Behavior & Withdrawal Symptoms
Common Abilify (aripiprazole) side effects include nausea, vomiting, headache, insomnia and weight gain. Serious Abilify side effects include tardive dyskinesia and neuroleptic malignant syndrome. Stopping Abilify may cause withdrawal. Abilify withdrawal symptoms include anxiety, panic attacks and sweating.
Serious Abilify side effects include tardive dyskinesia and neuroleptic malignant syndrome. Stopping Abilify may cause withdrawal. Abilify withdrawal symptoms include anxiety, panic attacks and sweating.
Common Abilify Side Effects
Common side effects of Abilify include nausea, vomiting, dizziness, anxiety, insomnia, constipation, movement disorders and restlessness. The most common side effect is headaches.
Abilify, also known as aripiprazole, is an atypical antipsychotic medication that doctors prescribe for major depressive disorder, bipolar I disorder and schizophrenia, and to manage irritability from autism spectrum disorder or Tourette’s syndrome in children.
Other common side effects of Abilify include:
- Blurred vision
- Drooling
- Drops in blood pressure when standing up
- Constipation
- Choking or trouble swallowing
Sleepiness is a common Abilify side effect for children ages 6 to 18. Other side effects for children include headache, nausea, vomiting, insomnia, movement disorders, increased appetite and weight gain.
Other side effects for children include headache, nausea, vomiting, insomnia, movement disorders, increased appetite and weight gain.
If the drug does not reduce psychiatric symptoms, consider asking your doctor to switch you to a different antipsychotic medication.
Serious Side Effects of Abilify
Abilify can cause serious side effects, many of which can be dangerous to long-term physical and mental health. These include seizures, involuntary muscle movements and neuroleptic malignant syndrome. The drug is also associated with harmful metabolic changes, including weight gain, increased cholesterol, low white blood cell counts, insulin resistance and nonalcoholic fatty liver disease.
Abilify can cause new and worsening suicidal thoughts and impulse control disorders. People experiencing impulse control while taking Abilify might engage in behaviors such as compulsive gambling, hypersexuality, compulsive shopping and spontaneous wandering. Recent reports suggest Abilify might also cause obsessive-compulsive thoughts and behaviors.
Black Box Warning for Elderly Dementia Patients
Abilify’s drug label includes a black box warning stating that elderly people with dementia who take the drug are at an increased risk of death. In clinical trials, elderly people with dementia taking atypical antipsychotics such as Abilify were 1.7 times more likely to die than those in the control group.
Clinical data does not spell out the reason for this increased mortality risk, but suggests the problem is caused by multiple factors. Elderly people who take antipsychotics such as Abilify are more likely to suffer strokes, which are often fatal within this population. They may also be more vulnerable to cardiovascular events such as heart attacks and serious infections such as pneumonia.
Doctors normally prescribe Abilify for elderly patients with dementia when there is a serious risk the person will harm themselves or others. In some cases, the risks are necessary to prevent greater harm. If you or a loved one with dementia have a prescription for Abilify, take it exactly as directed by your doctor.
Neuroleptic Malignant Syndrome
Neuroleptic malignant syndrome is a rare, life-threatening reaction to antipsychotic drugs such as Abilify. It is a potential side effect of almost all antipsychotic drugs.
Characteristics of NMS include fever, altered mental status, muscle rigidity and instability of the autonomic nervous system. This system controls unconscious functions such as breathing, heart rate, digestion, urination and sexual arousal.
Tardive Dyskinesia (Uncontrolled Body Movements)
Tardive dyskinesia, another serious side effect of Abilify, is characterized by involuntary muscle movements throughout the body, but mostly in the lower face.
TD is most common among people who take Abilify for months or years, but it can manifest as soon as six weeks after beginning the drug. TD can go away after you stop taking Abilify, but sometimes it becomes a permanent condition.
Abilify and Weight Gain
Studies also link Abilify to weight gain, particularly in children. A 2022 review in Australasian Psychiatry examined 11 studies and found that young people (mean age 18) gained an average of 2.7 kg (6 pounds) while on aripiprazole. Youths who took the drug for longer periods (more than 12 weeks) gained more weight than those who took it for shorter periods. Dosage sizes had no impact on weight gain.
A 2022 review in Australasian Psychiatry examined 11 studies and found that young people (mean age 18) gained an average of 2.7 kg (6 pounds) while on aripiprazole. Youths who took the drug for longer periods (more than 12 weeks) gained more weight than those who took it for shorter periods. Dosage sizes had no impact on weight gain.
Abilify sometimes causes weight gain in adults. This happens less often than it does in children, and the total weight gain is usually less significant. Studies suggest that aripiprazole is less likely to cause weight gain in adults than most other antipsychotics in use today.
Abilify Withdrawal
Because Abilify affects how your brain works, stopping the drug all at once may lead to withdrawal symptoms. This is more likely when you quit the drug after long-term use or if you are used to taking high doses of the medication.
Abilify withdrawal symptoms include:
- Anxiety
- Appetite changes
- Concentration problems
- Confusion
- Depression
- Diarrhea
- Dizziness
- Hallucinations
- Headache
- Joint pain
- Panic attacks
- Sweating
- Vomiting
Experts recommend tapering off Abilify if you want to stop taking it. Always consult your doctor for correct dosages when weaning yourself off of the drug.
Always consult your doctor for correct dosages when weaning yourself off of the drug.
Impulse Control Issues from Abilify
In 2014, a study published in JAMA Internal Medicine found that dopamine receptor agonist drugs such as Abilify were associated with new and worsening impulse control disorders. This research led to a new warning on the drug’s label, mandated by the U.S. Food and Drug Administration. This warning cautions that the drug may cause serious impulse control problems such as problem gambling, compulsive shopping and binge eating.
A 2022 review of impulse control disorder reports uploaded to the FDA Adverse Event Reporting System since December 2020 confirms that this association is still ongoing. About 94% of the impulse control disorder reports examined during the study involved people taking aripiprazole.
Many people who feel their lives were irreparably altered from gambling or uncontrolled impulse spending after taking the drug filed Abilify lawsuits to seek compensation.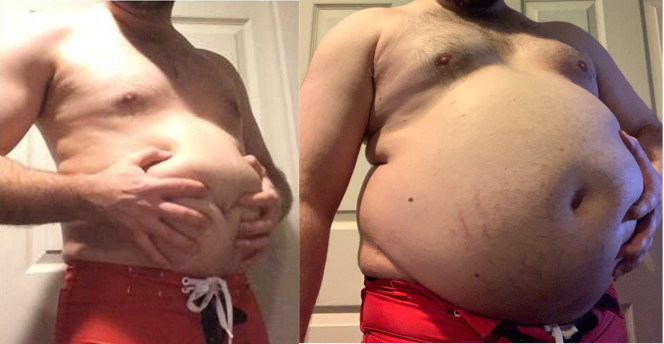
Impulse control problems related to Abilify often go away when you stop taking the drug or reduce your dose. If you struggle with impulse control, talk to your doctor about reducing the dose or switching medications. Don’t lower your dosage or stop taking your medication without informing your doctor.
Compulsive Gambling
Problem gambling is the most common impulse control-related side effect of Abilify. The medication may be more likely to cause gambling problems than other related drugs with similar impulse control effects. A 2021 study of Swedish health records found that people taking Abilify were significantly more likely to develop problem gambling behaviors than people taking other dopamine agonists.
Most of those who develop gambling issues while taking Abilify have no prior history of problem gambling. Contact your doctor immediately if you have the urge to gamble more frequently than usual.
Sexual Side Effects of Abilify
Hypersexuality is another manifestation of Abilify’s effects on impulse control. This includes having unprotected sex, compulsive masturbation, having sex with multiple partners, or having sex outside of a committed relationship. Some people engage in sexting or share explicit images of themselves.
This includes having unprotected sex, compulsive masturbation, having sex with multiple partners, or having sex outside of a committed relationship. Some people engage in sexting or share explicit images of themselves.
FDA data shows that 34% of people affected by antipsychotic-related impulse control disorders display uncharacteristic hypersexual thoughts or behaviors. Like Abilify’s other impulse control side effects, hypersexual behavior usually stops when you stop taking the drug or lower your dose.
Suicidal Thoughts
People who take Abilify have an increased risk of developing suicidal thoughts and behaviors. For those who already experience these issues, Abilify may worsen them. The drugmaker now notes this side effect in the black box warning on the drug’s label.
Monitor yourself for new or worsening suicidal thoughts while taking Abilify. Contact your doctor immediately if you notice them. Don’t stop taking your medication without your doctor’s knowledge.
Aripiprazole Side Effects
Please seek the advice of a medical professional before making health care decisions.
TELL US WHAT YOU THINK
Did You Find Drugwatch Helpful?
Yes No
Thank you for your feedback. Do you have any thoughts you'd like to share about Drugwatch.com?
This article changed my life!
This article was informative
I have a question
How can we improve this page?
This article contains incorrect information
This article doesn't have the information I'm looking for
I have a question
How can we improve this page?
Thank You for Your Feedback
We appreciate your feedback. One of our content team members will be in touch with you soon.
We appreciate your feedback. One of our content team members will be in touch with you soon.
Cariprazine use and likelihood of weight gain
- In clinical trials, weight gain in patients treated with cariprazine was comparable to weight gain with placebo 1 .
- Significant weight gain may occur in some patients while taking cariprazine and should be carefully monitored. 1 .
- Cariprazine is metabolically neutral: the incidence of hyperlipidemia, hyperglycemia and diabetes mellitus in clinical studies was comparable to the incidence of these events when using placebo 2-4 .
In this section:
Antipsychotics may cause weight gain
People with schizophrenia are more likely to be overweight or obese than the general population 5 . During therapy with antipsychotic drugs, most patients may experience a clinically significant increase in body weight. Weight gain is a problem for patients because it can contribute to the development of type 2 diabetes, dyslipidemia and hypertension, which increases the risk of cardiovascular events 6 .
Weight gain is a problem for patients because it can contribute to the development of type 2 diabetes, dyslipidemia and hypertension, which increases the risk of cardiovascular events 6 .
Although diet and a sedentary lifestyle also contribute to weight problems in patients with schizophrenia, strong evidence suggests that antipsychotics are a key factor in weight gain 5.7 . This side effect is different for each antipsychotic drug. But no remedy works equally for all treated patients 8 . The incidence of clinically significant weight gain (≥7%) associated with treatment with atypical antipsychotics has been reported in the range of 8.1% (aripiprazole) 9 up to 22.2% (olanzapine) 10 .
Advice for clinicians and patients on managing weight gain
Because antipsychotics, including cariprazine, may be associated with weight gain, weight should be monitored in all patients with schizophrenia 1 . Body mass index (BMI) is considered the accepted standard for deciding whether to correct overweight 5 .
Body mass index (BMI) is considered the accepted standard for deciding whether to correct overweight 5 .
The patient's waist circumference can also be an informative indicator of pathological weight gain. Women with a waist circumference ≥90 cm and men with a waist circumference ≥100 cm have an increased risk of high blood pressure, type 2 diabetes, dyslipidemia, and metabolic syndrome 11 .
| IMT 12,13 | Wessor |
| 18.5-24.9 | normal weight |
| 25.0-29.9 | Overweight |
| 30 or more | Obesity |
the patient to a doctor within the first 6 months after starting therapy or changing the drug 5 . If values are stable, weight and BMI should be checked quarterly in normal weight patients and more frequently in overweight patients. nine0030 Patients who visit more than 1 month apart should be educated about weight management and asked to come in off-schedule if they begin to gain weight 5 .
nine0030 Patients who visit more than 1 month apart should be educated about weight management and asked to come in off-schedule if they begin to gain weight 5 .
If the patient is gaining weight, counsel on dietary changes, exercise choices, use of weight loss medications, and consider switching from an antipsychotic to an agent that is less likely to cause weight gain 14.15 .
Methods for preventing weight gain
As with other antipsychotics, weight gain has been observed in some patients with cariprazine 1 . Studies have shown that when taking cariprazine, a weight gain of 1 kg occurred, both in short-term and long-term treatment 1 . In a long-term study using dosages of 1.5 mg-6 mg/day, it was shown that weight gain was recorded in 9.0% of patients taking cariprazine 1 . Compared with the placebo group, an increase in body weight was recorded in 5.1% of patients receiving cariprazine and 1. 5% of patients receiving placebo 16 .
5% of patients receiving placebo 16 .
In general, it can be said that cariprazine leads to a slight increase in body weight 1 .
| Placebo n=683 | Cariprazine 1.5-6 mg n=2048 | |
| Weight gain 1 | 0.9 kg | 1.1 kg |
| Age weight ≥7% 1 | 7.1% | 9.8% |
Metabolic changes using carpentry Cariprazine does not appear to have a clinically significant adverse effect on metabolic parameters.
Mean (SD) changes in metabolic characteristics from baseline with authorized doses of cariprazine were small and clinically insignificant 4 .
The number of patients who experienced adverse events associated with hyperlipidemia was approximately 1% in all treatment groups. The number of patients who experienced adverse events associated with hyperglycemia and diabetes mellitus was <1% in the cariprazine treatment group and 1% in the placebo group 16 .
In short-term studies, changes from normal to high lipid and glucose levels were generally comparable between placebo and cariprazine 2 .
In conclusion, the results of the study indicate that cariprazine may be considered an acceptable treatment option for patients with schizophrenia at risk of developing cardiovascular disease due to problems with excess weight or metabolic disorders 2-4 .
Instructions for medical use of Reagila® RU: LP-005405 dated 03/18/2019. The instructions can be found on the website: www.grls.rosminzdrav.ru.
Sources:
- Reagila SmPC. Instructions for medical use of Reagila® RU: LP-005405 dated 03/18/2019.
- Earley, W. et al. Safety and tolerability of cariprazine in patients with acute exacerbation of schizophrenia: A pooled analysis of four phase II/III randomized, double-blind, placebo-controlled studies. Int. Clin. Psychopharmacol. 32, 319–28 (2017).
- Leucht, S.
 et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: Systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am. J. Psychiatry 174, 927–942 (2017).
et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: Systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am. J. Psychiatry 174, 927–942 (2017). - Citrome, L. Cariprazine in schizophrenia: Clinical efficacy, tolerability, and place in therapy. Adv. Ther. 30, 114–126 (2013).
- Marder, S. R. et al. Physical health monitoring of patients with schizophrenia. Am. J. Psychiatry 161, 1334–1349 (2004).
- Kapur, S. & Marques, T. R. Dopamine, striatum, antipsychotics, and questions about weight gain. JAMA Psychiatry 73, 107–108 (2016).
- Padmavati, R., McCreadie, R. G. & Tirupati, S. Low prevalence of obesity and metabolic syndrome in never-treated chronic schizophrenia. Schizophr. Res. 121, 199–202 (2010).
- Musil, R., Obermeier, M., Russ, P. & Hamerle, M. Weight gain and antipsychotics: A drug safety review. Expert Opin. drug safe. 14, 73–96 (2015).
- Abilify [package insert].
 Rockville, MD: Otsuka America Pharmaceutical, Inc https://www.otsuka-us.com/media/static/Abilify-PI.pdf (2018).
Rockville, MD: Otsuka America Pharmaceutical, Inc https://www.otsuka-us.com/media/static/Abilify-PI.pdf (2018). - Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC https://pi.lilly.com/us/zyprexa-pi.pdf (2018).
- Janssen, I., Katzmarzyk, P. T. & Ross, R. Body Mass Index, Waist Circumference, and Health Risk. Arch. Intern. Med. 162, 2074–2079(2002).
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Heal. Organ. – Tech. Rep. Ser. (2000).
- National Heart Lung and Blood Institute & National Institutes of Health (NIH) National Heart, Lung, and Blood Institute, N. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. The Evidence Report, NIH Publication No. 98-4083. WMJ official publication of the State Medical Society of Wisconsin (1998) doi:10.1001/jama.2012.39.
- Aquila, R. Management of weight gain in patients with schizophrenia. J.Clin. Psychiatry 63, 33–36 (2002).

- Ball, M. P., Coons, V. B. & Buchanan, R. W. A program for treating olanzapine-related weight gain. Psychiatr. Serv. 52, 967–969 (2001).
- EMA. Reagila Assessment Report. https://www.ema.europa.eu/en/documents/assessment-report/reagila-epar-public-assessment-report_en.pdf.
Advances in Therapy
Treatment with cariprazine: clinical efficacy, tolerability
Citrome L.
Adv Ther. 2013; 30(2):114–26.
"Potential candidates for treatment with cariprazine may include adult patients with schizophrenia for whom the risk of metabolic or cardiovascular disease is a concern, as well as patients in whom weight gain should be minimized or prevented."
Login to Unlock
Mechanism of action of Reagila Mechanism of action of Reagila
(Reagila® RU: LP-005405 dated 03/18/2019) The therapeutic effect of cariprazine is provided by a combination of partial agonism in relation to dopamine D3- and D2-recLearn more about the mechanism of action of Reagila
More…
Login to Unlock
Go to treatment with Reagi… Switching to treatment with Reagi…
(Reagila® RU: LP-005405 dated 03/18/2019) patient, maintaining and returning self-service skills, social interaction
Next…
Please confirm your consent to the use of cookies
Yes, I agree
No, I refuse
Please confirm that you are a health worker
Showing 0 result(s).
Please log in to see 0 more result(s).
LONG-ACTING ANTIPSYCHOTIC USE IN SCHIZOPHRENIA | Bibekova
Introduction
Schizophrenia is an endogenous polymorphic mental disorder characterized by the breakdown of thought processes and emotional reactions. [1][2]. More than 21 million people suffer from it worldwide.
The symptoms of schizophrenia are divided into the following categories: positive, negative and cognitive. Positive symptoms include hallucinations, delusions, thought disturbances, and movement disorders.
Negative symptoms include apathy, abulia and impoverishment of speech. Cognitive symptoms include impaired ability to comprehend information, trouble concentrating or shifting attention, and memory problems. Factors that may influence the occurrence of schizophrenia include genetic predisposition, exposure to viruses, environmental factors, problems during childbirth, and psychosocial factors [3]. However, there is no specific characteristic of schizophrenia, making it difficult for clinicians to make an accurate diagnosis based on symptoms or laboratory tests. For example, there was evidence that the clinical symptoms of schizophrenia, such as cognitive impairment, emotional instability, personality changes, delusions, hallucinations, and inappropriate behavior, were similar to the symptoms of general paresis in neurosyphilis [4]. nine0024
For example, there was evidence that the clinical symptoms of schizophrenia, such as cognitive impairment, emotional instability, personality changes, delusions, hallucinations, and inappropriate behavior, were similar to the symptoms of general paresis in neurosyphilis [4]. nine0024
Epidemiology
The prevalence of schizophrenia in the world is 1% of the total population [5]. The average life expectancy of people suffering from schizophrenia in developed countries is about 20 years less than in the general population, but these data are influenced by the age of onset of schizophrenia and gender [6][7].
The leading causes of premature death in people with schizophrenia are cardiovascular, respiratory and cancer diseases and injuries. Risk factors for the development of these conditions are late detection of oncological pathology, abuse of nicotine, alcohol and drugs, and the frequent presence of metabolic syndrome [8]. Suicide is one of the leading causes of death in schizophrenia [9]. ].
].
Schizophrenia is one of the top 15 causes of disability worldwide [10]. The average age of onset of schizophrenia is 15 to 30 years (20 to 30 years for women and 15 to 25 years for men) [11].
Problems in the treatment of schizophrenia
Antipsychotic drugs (neuroleptics) are most commonly used for schizophrenia, one of the most debilitating and serious mental illnesses. Antipsychotics allow a sick person to function normally in society. The mechanism of action of this group of drugs is based on the inhibition of β-adrenergic, histamine and muscarinic receptors. Starting from the second generation, antipsychotics also block serotonin receptors [12]. Antipsychotic drugs are the mainstay of treatment for schizophrenia at all stages. Antipsychotics provide significant benefits in the treatment of schizophrenia, but they are associated with a high risk of side effects, the main manifestations of which include anticholinergic effects, weight gain, extrapyramidal symptoms, agranulocytosis, and seizures. Adverse effects on medications and factors such as poor patient understanding of their disease, substance abuse, and inadequately scheduled discharges from medical organizations may contribute to patient medication disruption [13]. For many patients, a violation of the intake of pharmacological drugs is the reason for the onset of decompensation and relapse of the disease. In turn, relapses of schizophrenia lead to more frequent hospitalizations. Relapse and hospitalization are the main factors that add up to the cost of treating schizophrenia [14]. nine0024
Adverse effects on medications and factors such as poor patient understanding of their disease, substance abuse, and inadequately scheduled discharges from medical organizations may contribute to patient medication disruption [13]. For many patients, a violation of the intake of pharmacological drugs is the reason for the onset of decompensation and relapse of the disease. In turn, relapses of schizophrenia lead to more frequent hospitalizations. Relapse and hospitalization are the main factors that add up to the cost of treating schizophrenia [14]. nine0024
Schizophrenia is a chronic mental illness that requires lifelong maintenance therapy with antipsychotic drugs. Discontinuation of therapy, often due to poor adherence, increases the risk of relapse after the first and subsequent episodes of psychosis. Long-acting antipsychotics have been introduced to increase therapeutic adherence, reduce the variability in blood levels of the drug compared to the corresponding oral forms [15]. nine0024
nine0024
Long-acting antipsychotics
Haloperidol decanoate
Haloperidol is a first-generation antipsychotic drug that is a derivative of butyrophenone. Haloperidol is lipophilic, which is easily absorbed and actively metabolized in the liver [16]. Haloperidol decanoate is a long-acting drug that has been used in clinical practice for many years [17].
In 2016, Taylor D.M., Sparshatt A., O’Hagan M. et al conducted a study comparing the efficacy and safety of haloperidol decanoate and paliperidone palmitate in 300 patients. The result of the study showed no noticeable difference in efficacy between the two treatments. Haloperidol decanoate was prescribed strictly at the therapeutic dose, and maintenance doses were lower than traditional practice. The frequency of side effects varied between the drugs, but the overall tolerability of the drugs was comparable. Side effects with the use of paliperidone were: an increase in body weight and an increase in the level of prolactin in the blood serum. Haloperidol has been associated with side effects such as akathisia [18]. nine0024
Haloperidol has been associated with side effects such as akathisia [18]. nine0024
In another double-blind, randomized clinical trial by McEvoy J.P., Byerly M., Hamer R.M. and others from March 2011 to July 2013 in 22 US clinical research centers comparing paliperidone palmitate versus haloperidol decanoate in adults with schizophrenia or schizoaffective disorder, there was no significant difference in drug efficacy. The results are supported by previous studies that did not find large differences in the effectiveness of new and old antipsychotics [19].
Mace S., Dzahini O., O'Hagan M. et al. in their study reported the following side effects of haloperidol decanoate: extrapyramidal side effects (62%), anxiety (14%), weight gain (10%), hypersalivation (10%), lack of sleep (5%), sexual dysfunction (5%), constipation (5%), urinary incontinence (5%), cessation of menstruation (5%), discomfort (5%), fatigue (5% ), pain (5%) injection site abscess (5%), skin allergy (5%), chest pain (5%), sedation (5%), decreased sensation in the hands (5%). Haloperidol decanoate was a common reason for stopping treatment. But at the same time, the number of hospitalizations during the year decreased after taking the drug, although there was no change in such an indicator as a bed-day. The study found that patients suffering from a longer duration of the disease and patients who started taking haloperidol decanoate for reasons other than poor adherence to treatment may benefit from treatment with a long-acting form of haloperidol [20]. nine0024
Haloperidol decanoate was a common reason for stopping treatment. But at the same time, the number of hospitalizations during the year decreased after taking the drug, although there was no change in such an indicator as a bed-day. The study found that patients suffering from a longer duration of the disease and patients who started taking haloperidol decanoate for reasons other than poor adherence to treatment may benefit from treatment with a long-acting form of haloperidol [20]. nine0024
Risperidone
Risperidone is the first new-generation antipsychotic drug available as a long-acting injection [21].
Mihaljevic-Peles A., Sagud M., Filipcic I.S. et al conducted a 1-year prospective study to investigate the efficacy of long-acting risperidone in patients with schizophrenia for endpoints such as remission rate and hospitalization status. The study involved 362 patients with a mean age of 37 years. The efficacy and safety of treatment was assessed using scales such as CGI (1976 US National Institute of Mental Health), PANSS (1986, Kay S. R., Opler L.A., Fiszbein A.). After 12 months, the following data were obtained: in patients treated with long-acting risperidone, there were significant improvements in CGI scores, hospitalization status, and remission rates. Data point to the benefits of continuous treatment [22].
R., Opler L.A., Fiszbein A.). After 12 months, the following data were obtained: in patients treated with long-acting risperidone, there were significant improvements in CGI scores, hospitalization status, and remission rates. Data point to the benefits of continuous treatment [22].
Long-acting risperidone is well tolerated by patients with schizophrenia. Only 88 of 1491 patients (6%) who received the drug for 6 months reported at least one of the side effects (anxiety, insomnia, depression, headache) [23]. nine0024
Paliperidone palmitate
Paliperidone palmitate is a long-acting antipsychotic used to treat patients with schizophrenia with poor adherence to treatment. The drug is maintained at a therapeutic dose in plasma for four weeks [24]. Paliperidone palmitate has been shown to be highly effective in reducing psychotic symptoms, improving functional status and preventing relapses, and significantly reducing hospitalizations [25]. nine0024
Li N., Feng Y., Lu H. et al. conducted a study on the efficacy of symptomatic and functional remission in patients with schizophrenia who switched from oral antipsychotics to once-monthly injections of paliperidone palmitate. Patients treated with 75 to 150 mg of paliperidone palmitate showed an improvement in PANSS scores at week 5 [26].
et al. conducted a study on the efficacy of symptomatic and functional remission in patients with schizophrenia who switched from oral antipsychotics to once-monthly injections of paliperidone palmitate. Patients treated with 75 to 150 mg of paliperidone palmitate showed an improvement in PANSS scores at week 5 [26].
In a six-week study of the efficacy and safety of paliperidone palmitate, the following adverse reactions were identified: tachycardia (9-22%), hyperkinesis (3-11%), hypertension (1-6%), extrapyramidal symptoms (3-10%) and drowsiness (4-13%) [27].
In another 18-month study of the drug, conducted by Si T., Zhuo J., Feng Y. et al., in which 118 patients participated, the following side effects were identified: insomnia (13.9%), pain in the injections (13.9%), upper respiratory infection (13.0%), anxiety (13.0%), and akathisia (13.0%). One case of suicide has also been reported [28].
A new form of paliperidone palmitate is available that maintains the therapeutic dosage of the drug for 3 months. The drug needs to be administered only 4 times a year [29]. Paliperidone palmitate 3-monthly is approved for use in the US for the treatment of schizophrenia only after adequate treatment with monthly paliperidone palmitate for > 4 months. In a double-blind, placebo-controlled study, data were obtained that patients with early clinically significant improvements in symptoms and disease severity when a stable dose of the drug is established, maintaining a therapeutic dosage for a month, are more likely to achieve remission after a subsequent switch to the drug form with maintaining a therapeutic dosage for three months [30]. nine0024
The drug needs to be administered only 4 times a year [29]. Paliperidone palmitate 3-monthly is approved for use in the US for the treatment of schizophrenia only after adequate treatment with monthly paliperidone palmitate for > 4 months. In a double-blind, placebo-controlled study, data were obtained that patients with early clinically significant improvements in symptoms and disease severity when a stable dose of the drug is established, maintaining a therapeutic dosage for a month, are more likely to achieve remission after a subsequent switch to the drug form with maintaining a therapeutic dosage for three months [30]. nine0024
In 2018 Mathews M., Nuamah I., Savitz A.J. et al. conducted a study evaluating the occurrence of extrapyramidal disorders in the 3-month form of paliperidone palmitate compared to the monthly form. Despite differences in apparent half-life and pharmacokinetic profiles, the drugs showed a comparable incidence of extrapyramidal disorders in patients with schizophrenia [31].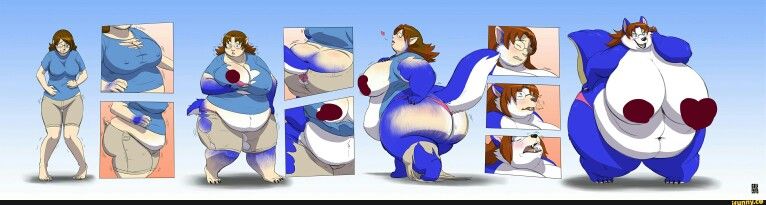 The most common side effects that occurred when switching from a monthly form of paliperidone palmitate to a three-month form were headache and nasopharyngitis, weight gain and back pain were less common [32]. nine0024
The most common side effects that occurred when switching from a monthly form of paliperidone palmitate to a three-month form were headache and nasopharyngitis, weight gain and back pain were less common [32]. nine0024
Aripiprazole lauroxyl
Aripiprazole lauroxyl is a long-acting drug used to treat schizophrenia in adults [33].
In 2017, McEvoy J.P., Risinger R., Mykhnyak S. et al conducted a study to evaluate the duration of therapeutic effect after long-acting treatment with aripiprazole lauroxil in patients with schizophrenia after successful treatment of an acute psychotic episode. Efficacy was assessed by PANSS, CGI-S scores and the proportion of patients who completed a one-year course of therapy. The results of the study demonstrate the therapeutic efficacy of aripiprazole lauroxyl after successful treatment of an acute episode of schizophrenia. Both groups of 441 mg and 882 mg showed the same efficacy results on the PANSS, CGI-S scales and in the probability of remission [34]. The drug can be administered every 8 weeks at a dosage of 1064 mg, which is confirmed by the study of its pharmacokinetics [35]. A review of the literature comparing the efficacy and safety of aripiprazole lauroxyl and paliperidone palmitate showed that there were no significant differences in the treatment of both drugs in patients with acute schizophrenic attacks [36]. nine0024
The drug can be administered every 8 weeks at a dosage of 1064 mg, which is confirmed by the study of its pharmacokinetics [35]. A review of the literature comparing the efficacy and safety of aripiprazole lauroxyl and paliperidone palmitate showed that there were no significant differences in the treatment of both drugs in patients with acute schizophrenic attacks [36]. nine0024
In 2019, Nasrallah H.A., Aquila R., Du Y. et al. conducted a 52-week multicentre safety study of aripiprazole lauroxil in patients with schizophrenia, which included 478 patients. Scientists studied the drug in two fixed dosages: 441 and 882 mg. The end points by which the safety of the drug was assessed were extrapyramidal symptoms, significant changes in endocrine and metabolic parameters, as well as other side effects. The study involved 478 patients divided into three groups: I - with a dosage of 441 mg, II - with a dosage of 882 mg and III - with placebo. The most common side effects were insomnia (8. 4%) and weight gain (5.0%). Akathisia was reported in 3.8% of patients. Extrapyramidal disorders - in 9.4% of patients, and side effects associated with metabolic parameters were observed in 4.6% of patients. Weight gain was minimal (0.8 kg) and no clinically significant changes in metabolic parameters were noted [37].
4%) and weight gain (5.0%). Akathisia was reported in 3.8% of patients. Extrapyramidal disorders - in 9.4% of patients, and side effects associated with metabolic parameters were observed in 4.6% of patients. Weight gain was minimal (0.8 kg) and no clinically significant changes in metabolic parameters were noted [37].
Fluphenazine decanoate
Fluphenazine is a tricyclic phenothiazine that acts by postsynaptic inhibition of dopamine receptors in the mesolimbic, nigrostriatal, and tuberoinfundibular nerve pathways [38].
Zhao Y.J., Lin L., Teng M. et al. conducted a meta-analysis of antipsychotic monotherapy aimed at reducing the risk of relapse in schizophrenia. The meta-analysis included 10,177 patients. The following results were obtained: fluphenazine decanoate was more effective than chlorpromazine in reducing the recurrence of schizophrenia. Fluphenazine decanoate, haloperidol, haloperidol decanoate, and trifluoperazine frequently caused extrapyramidal side effects [39].
Maayan N., Quraishi S.N., David A. conducted a study on the efficacy and safety profile of fluphenazine decanoate and enanthate. No study comparing fluphenazine decanoate with placebo reported clinically significant changes in general condition or hospitalizations. Fluphenazine decanoate does not reduce relapse to a greater extent than oral antipsychotics in the medium term. Extrapyramidal side effects were significantly less common in patients treated with fluphenazine decanoate compared with oral antipsychotics [40]. nine0024
Zuclopenthixol decanoate
Coutinho E., Fenton M., Quraishi S. conducted a study to collect information on randomized trials of long-term forms of zuclopenthixol comparing the efficacy and safety of zuclopenthixol decanoate, oral zuclopenthixol and other antipsychotic drugs for the treatment of schizophrenia and similar serious mental illnesses. Scientists have obtained evidence that zuclopenthixol decanoate prevents or delays relapses compared to other depot preparations.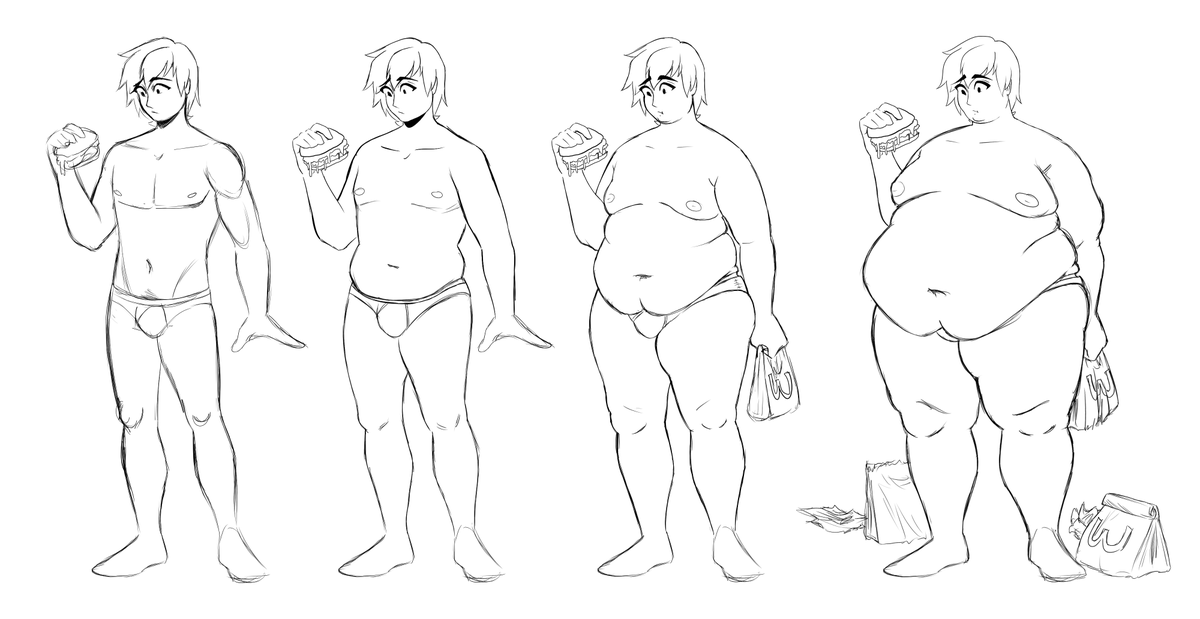 But at the same time, side effects were more common in this group. The authors of the study state the need for a study to evaluate the economic and clinical effectiveness of the use of zuclopenthixol decanoate. The duration of such trials should be longer than the included studies (12 months or more) [41]. nine0024
But at the same time, side effects were more common in this group. The authors of the study state the need for a study to evaluate the economic and clinical effectiveness of the use of zuclopenthixol decanoate. The duration of such trials should be longer than the included studies (12 months or more) [41]. nine0024
Flupenthixol decanoate
Mahapatra J., Quraishi S.N., David A. et al reviewed 15 randomized controlled trials with 626 participants. None of the studies compared flupenthixol decanoate with placebo. One small study compared flupenthixol decanoate with an oral antipsychotic (Penfluridol). This study did not show a clear difference between the two drugs in terms of early exit from the study and the need for anticholinergic therapy. Ten studies in total compared flupentixol decanoate with other depot formulations. There were no significant differences between the depot preparations in terms of short term clinical improvement, in the number of participants leaving the study early in the short/medium term, or in the number of people requiring anticholinergic treatment in the short/medium term. Three studies compared high doses (100-200 mg) of flupenthixol decanoate with standard doses (~40 mg) per injection. Two studies found that relapses in the medium term were similar between the groups. However, people who received the high dose had somewhat more favorable mid-term mental health outcomes, on the Short Psychiatric Rating Scale (BPRS). There were also no significant differences in the use of anticholinergic drugs to treat side effects in the short term. One study comparing a very low dose of flupentixol decanoate (6 mg) with a low dose (9mg) per injection, no difference in recurrence rates was reported [42].
Three studies compared high doses (100-200 mg) of flupenthixol decanoate with standard doses (~40 mg) per injection. Two studies found that relapses in the medium term were similar between the groups. However, people who received the high dose had somewhat more favorable mid-term mental health outcomes, on the Short Psychiatric Rating Scale (BPRS). There were also no significant differences in the use of anticholinergic drugs to treat side effects in the short term. One study comparing a very low dose of flupentixol decanoate (6 mg) with a low dose (9mg) per injection, no difference in recurrence rates was reported [42].
Long-acting olanzapine
Olanzapine is an atypical antipsychotic first approved by the US Food and Drug Administration (FDA) in September 1996 for the treatment of schizophrenia [43].
Fefeu M., De Maricourt P., Cachia A. et al. conducted a one-year cost-effectiveness study of long-acting olanzapine in patients with severe schizophrenia. The end results were: hospital admissions, hospital stays, outpatient visits, and inpatient cost estimates.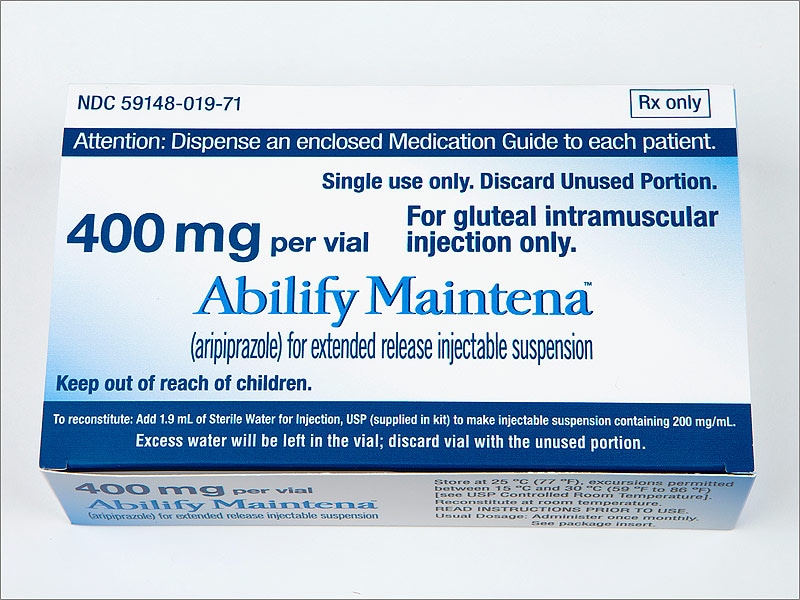 After one year, the use of long-acting olanzapine was associated with significant reductions in hospital admissions, bed-days, and inpatient costs. There were no significant differences in the number of outpatient visits and the incidence of side effects [44]. nine0024
After one year, the use of long-acting olanzapine was associated with significant reductions in hospital admissions, bed-days, and inpatient costs. There were no significant differences in the number of outpatient visits and the incidence of side effects [44]. nine0024
Kane J.M., Detke H.C., Naber D. conducted a 24-week, randomized, double-blind trial of maintenance therapy in patients with schizophrenia. Patients who were stable on oral olanzapine at doses of 10, 15, or 20 mg/day for 4 to 8 weeks were randomly assigned to different doses of the drug orally and by injection. The study provided evidence that the efficacy of maintenance therapy for schizophrenia is similar between oral and injectable olanzapine. No significant differences were observed in the safety profile between injectable and oral olanzapine. Two patients had sedation and delirium, consistent with an overdose of the drug after a possible accidental intravascular injection [45]. nine0024
Conclusion
Long-acting antipsychotics have been shown to be more effective than oral formulations in reducing hospitalizations and prolonging remission. The safety profile of injectable and oral forms of antipsychotics is on the same level.
The safety profile of injectable and oral forms of antipsychotics is on the same level.
There was no significant difference in efficacy between haloperidol decanoate, paliperidone palmitate, and aripyrazole lauroxyl.
Risperidone showed the best safety profile among the presented long-acting antipsychotics. nine0024
Fluphenazine decanoate and long-acting olanzapine have shown efficacy and safety similar to oral formulations.
Zuclopenthixol decanoate showed greater efficacy compared to other depot preparations. But its use is associated with a high incidence of side effects.
Flupenthixol decanoate has only been shown to be effective at high doses, making it difficult to use.
1. Karam C.S., Ballon J.S., Bivens N.M., Freyberg Z., Girgis R.R., et al. Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. // Trends Pharmacol Sci. – 2010. – V.31(8). – P.381–90. doi: 10.1016/j.tips.2010.05.004
2. Xu Y., Ren J. , Ye H. Association between variations in the disrupted in schizophrenia 1 gene and schizophrenia: A metaanalysis. // Gene. - 2018. - V.651. – P.94–99. doi: 10.1016/j. gene.2018.01.069.
, Ye H. Association between variations in the disrupted in schizophrenia 1 gene and schizophrenia: A metaanalysis. // Gene. - 2018. - V.651. – P.94–99. doi: 10.1016/j. gene.2018.01.069.
3. National Institute of Mental Health Schizophrenia. Available online: https://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml. (accessed on 1 February 2016)
4. Zhong X., Shi H., Hou L., Chen B., Peng Q., et al. Neuropsychiatric features of Neurosyphilis: frequency, relationship with the severity of cognitive impairment and comparison with Alzheimer disease. // Dement Geriatr Cogn Disord. – 2017. – V.43(5–6). – P.308–319. doi: 10.1159/000476060.
5. Owen M.J., Sawa A., Mortensen P.B. Schizophrenia. // Lancet. – 2016. – V.388(10039). – P.86–97. doi: 10.1016/S01406736(15)01121-6
6. Laursen T.M. Life expectancy among persons with schizophrenia or bipolar affective disorder. // Schizophre Res. – 2011. –
7. V.131(1–3). – P.101–4. doi: 10.1016/j.schres.2011.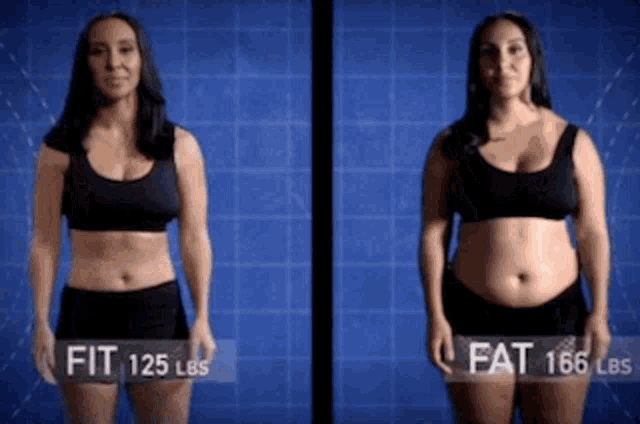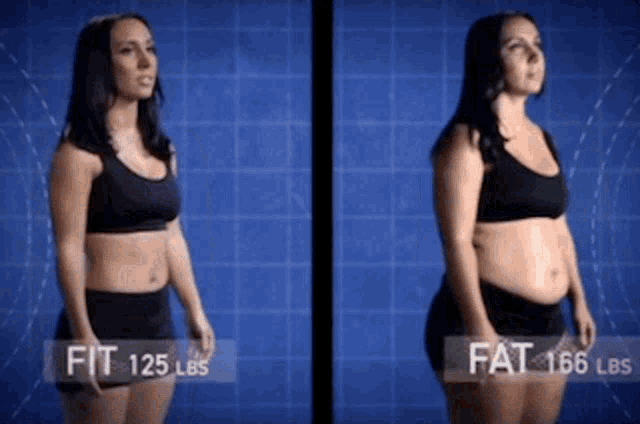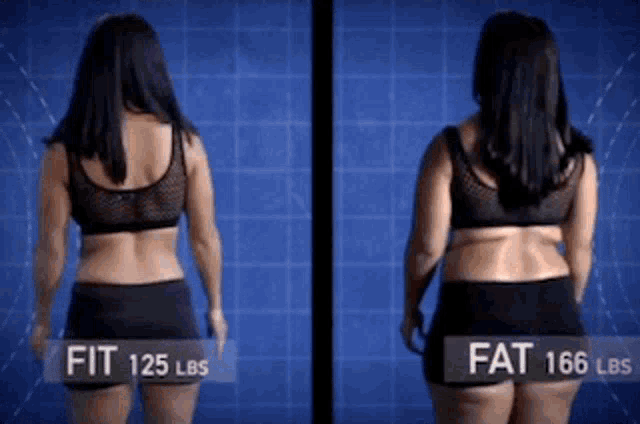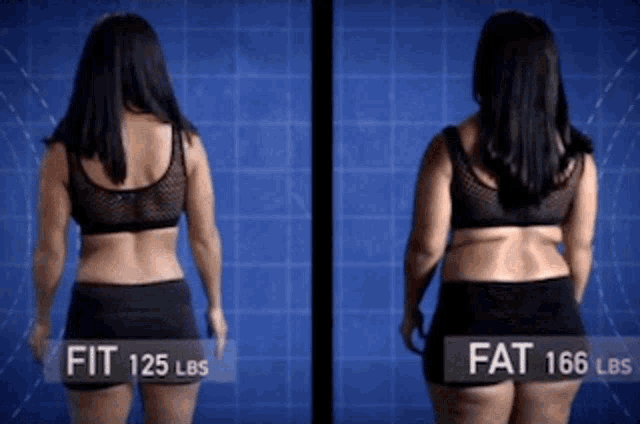 06.008
06.008
8. Leng C.H., Chou M.H., Lin S.H., Yang Y.K., Wang J.D. Estimation of life expectancy, loss-of-life expectancy, and lifetime healthcare expenditures for schizophrenia in Taiwan. // Schizophre Res. – 2016. – V.171(1–3). – P.97–102. doi: 10.1016/j.schres.2016.01.033
9. Olfson M., Gerhard T., Huang C., Crystal S., Stroup T.S. Premature Mortality Among Adults With Schizophrenia in the United States. // JAMA Psychiatry. – 2015. – V.72(12). – P.1172–81. doi: 10.1001/jamapsychiatry.2015.1737
10. Walker E.R., McGee R.E., Druss B.G. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. // JAMA Psychiatry. - 2015. - V.72(4). – P.334–41. doi: 10.1001/jamapsychiatry.2014.2502
11. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the global burden of disease study 2016. // Lancet. -
// Lancet. -
12. - V.390(10100). – P.1211–1259. doi: 10.1016/S01406736(17)32154-2. 11. Robertson L. Mental Health Matters. Volume 4. - Health Service; Gauteng, South Africa; 2017.
13. Stringer J.L. Antipsychotics or Neuroleptics. In: Stringer J.L., ed. Basic Concepts in Pharmacology: What You Need to Know for Each Drug Class. 5th ed. McGraw-Hill; New York, NY, USA: Available at: http://accessmedicine.mhmedical.com/content.aspx?bookid=2147§ionid=161351718. [accessed on March 7, 2019].
14. Lacro J.P., Dunn L.B., Dolder C.R., Leckband S.G., Jeste D.V. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. // J Clin Psychiatry. - 2002. - V.63(10). – P.892–909. doi: 10.4088/jcp.v63n1007
15. Ascher-Svanum H., Zhu B., Faries D.E., Salkever D., Slade E.P., et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. // BMC Psychiatry. -2010. – V. 10. – P.2. doi: 10.1186/1471-244X-10-2. nine0024
10. – P.2. doi: 10.1186/1471-244X-10-2. nine0024
16. Di Lorenzo R., Ferri P., Cameli M., Rovesti S., Piemonte C. Effectiveness of 1-year treatment with long-acting formulation of aripiprazole, haloperidol, or paliperidone in patients with schizophrenia: a retrospective study in a real world clinical setting. // Neuropsychiatr Dis Treat. – 2019. – V.15. – P.183-198. doi: 10.2147/NDT.S189245.
17. Gabriel R., Wojtanowicz T., Farokhpay R., Bota R. Acute transaminitis after initial days of starting haloperidol. // Ment Illn. – 2019. – V.11(1). – P.8113. doi: 10.4081/mi.2019.8113.
18. European Medicines Agency. Haldol Decanoate and Associated Names: Assessment Report. 2017. Committee for Medicinal Products for Human Use (CHMP).) (EMA/217985/2017). Available at: http://www.ema.europa.eu [accessed on 7 March 2019].
19. Taylor D.M., Sparshatt A., O’Hagan M., Dzahini O. Effect of paliperidone palmitate on hospitalization in a naturalistic cohort - a four-year mirror image study. // Eur Psychiatry. - 2016. - V.37. – P.43–48. doi: 10.1016/j.eurpsy.2016.04.009
// Eur Psychiatry. - 2016. - V.37. – P.43–48. doi: 10.1016/j.eurpsy.2016.04.009
20. McEvoy J.P., Byerly M., Hamer R.M., Dominik R., Swartz M.S., et all. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. // JAMA. – 2014. – V.311(19). – P.1978-87. doi: 10.1001/jama.2014.4310.
21. Mace S., Dzahini O., O’Hagan M., Taylor D. Haloperidol decanoate long-acting injection (HDLAI): Results of a 1-year mirror-image study. // TherAdv Psychopharmacol. – 2018. –V.8(9). – P.241-249. doi: 10.1177/2045125318767587
22. Hori H., Katsuki A., Atake K., Yoshimura R. Effects of Continuing Oral Risperidone vs. Switching from Risperidone to Risperidone Long-Acting Injection on Cognitive Function in Stable Schizophrenia Patients: A Pilot Study. // Front Psychiatry. – 2018. – V.9. – P.74. doi:10.3389/fpsyt.2018.00074.
23. Mihaljevic-Peles A., Sagud M., Filipcic I.S., Grosic V., Pedisic I., Emsley R. Remission and employment status in schizophrenia and other psychoses: One-year prospective study in Croatian patients treated with risperidone long acting injection. // Psychiatr Danub. - 2016. - V.28(3). – P.263-272. PMID: 27658835 23. Giraud-Baro E., Dassa D., De Vathaire F., Garay R.P., Obeid J. Schizophrenia-spectrum patients treated with long-acting injectable risperidone in real-life clinical settings: functional recovery in remitted versus stable , non-remitted patients (the EVEREST prospective observational cohort study). // BMC Psychiatry. - 2016. - V.16. – P.8. doi: 10.1186/s12888-0160712-1. nine0024
Remission and employment status in schizophrenia and other psychoses: One-year prospective study in Croatian patients treated with risperidone long acting injection. // Psychiatr Danub. - 2016. - V.28(3). – P.263-272. PMID: 27658835 23. Giraud-Baro E., Dassa D., De Vathaire F., Garay R.P., Obeid J. Schizophrenia-spectrum patients treated with long-acting injectable risperidone in real-life clinical settings: functional recovery in remitted versus stable , non-remitted patients (the EVEREST prospective observational cohort study). // BMC Psychiatry. - 2016. - V.16. – P.8. doi: 10.1186/s12888-0160712-1. nine0024
24. Jang S., Woo J. Five month-persistent extrapyramidal symptoms following a single injection of paliperidone palmitate: a case report. // Clinical Psychopharmacology and Neuroscience. – 2017. – V.15(3). – P.288–291. doi: 10.9758/ cpn.2017.15.3.288
25. Emsley R., Parellada E., Bioque M., Herrera B., Hernando T., Garcia-Dorado M. Real-world data on paliperidone palmitate for the treatment of schizophrenia and other psychotic disorders: a systematic review of randomized and nonrandomized studies. // Int Clin Psychopharmacol. – 2018. –
// Int Clin Psychopharmacol. – 2018. –
26. V.33(1). – P.15–33. doi:10.1097/YIC.000000000000019
27. Li N., Feng Y., Lu H., Cai S. L., Zhuo J., et al. Factors related to improvement of symptoms, function, and caregiver burden in Chinese patients with schizophrenia after switching to paliperidone palmitate once-monthly from oral antipsychotics. // Neuropsychiatr Dis Treat. – 2018. – V.14. – P.825-837. doi: 10.2147/NDT.S158353
28. Fernández-Miranda J.J., Díaz-Fernández S. Tolerability of effective high doses of paliperidone palmitate in patients with severe resistant schizophrenia. // International Clinical Psychopharmacology. – 2017. - V32(1). - P6–12. doi: 10.1097/ YIC.0000000000000151
29. Si T., Zhuo J., Feng Y., Lu H., Hong D., Zhang L. Long-term efficacy and safety of paliperidone palmitate once-monthly in Chinese patients with recent- onset schizophrenia. // Neuropsychiatr Dis Treat. – 2019. – V.15. – P.1685-1694. doi: 10.2147/NDT.S191803
30. Mathews M., Gopal S., Nuamah I., Hargarter L., Savitz A.J., et al. Clinical relevance of paliperidone palmitate 3-monthly in treating schizophrenia. // Neuropsychiatr Dis Treat. – 2019. – V.15. – P.1365-1379. doi: 10.2147/NDT.S197225
Mathews M., Gopal S., Nuamah I., Hargarter L., Savitz A.J., et al. Clinical relevance of paliperidone palmitate 3-monthly in treating schizophrenia. // Neuropsychiatr Dis Treat. – 2019. – V.15. – P.1365-1379. doi: 10.2147/NDT.S197225
31. Nash A.I., Turkoz I., Savitz A.J., Mathews M., Kim E. Predictors of achieving remission in schizophrenia patients treated with paliperidone palmitate 3-month formulation. // Neuropsychiatr Dis Treat. – 2019. – V.15. – P.731-737. doi: 10.2147/NDT.S194264
32. Mathews M., Nuamah I., Savitz A.J., Hough D.W., Najarian D., et al. Time to onset and time to resolution of extrapyramidal symptoms in patients with exacerbated schizophrenia treated with 3-monthly vs once-monthly paliperidone palmitate. // Neuropsychiatr Dis Treat. – 2018. – V.14. – P.2807-2816. doi: 10.2147/NDT.S175364
33. Gopal S., Vermeulen A., Nandy P., Ravenstijn P., Nuamah I., et al. Practical guidance for dosing and switching from paliperidone palmitate 1 monthly to 3 monthly formulation in schizophrenia. // Current Medical Resident Opinion. – 2015. – V.31(11). – P.2043–2054. 34. Hard M.L., Wehr A.Y., Du Y., Weiden P.J., Walling D., von Moltke L. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. // J Clin Psychopharmacol. – 2018. – V.38(5). - P.435-441. doi: 10.1097/JCP.0000000000000921
// Current Medical Resident Opinion. – 2015. – V.31(11). – P.2043–2054. 34. Hard M.L., Wehr A.Y., Du Y., Weiden P.J., Walling D., von Moltke L. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. // J Clin Psychopharmacol. – 2018. – V.38(5). - P.435-441. doi: 10.1097/JCP.0000000000000921
35. McEvoy J.P., Risinger R., Mykhnyak S., Du Y., Liu C.C., Stanford A.D. Durability of Therapeutic Response With Long-Term Aripiprazole Lauroxil Treatment Following Successful Resolution of an Acute Episode of Schizophrenia. // J Clin Psychiatry. – 2017. – V.78(8). – P.1103-1109. doi: 10.4088/JCP.17m11625.
36. Risinger R., Hard M., Weiden P.J. A Phase-1 Study Comparing Pharmacokinetic and Safety Profiles of Three Different Dose Intervals of Aripiprazole Lauroxil. // Psychopharmacol Bull. – 2017. – V.47(3). – P.26-34. PMID: 28839337
37. Cameron C., Zummo J., Desai D.N., Drake C., Hutton B., Kotb A. Aripiprazole Lauroxil Compared with Paliperidone Palmitate in Patients with Schizophrenia: An Indirect Treatment Comparison. // ValueHealth. – 2017. – V.20(7). – P.876-885. doi: 10.1016/j.jval.2017.03.010
Aripiprazole Lauroxil Compared with Paliperidone Palmitate in Patients with Schizophrenia: An Indirect Treatment Comparison. // ValueHealth. – 2017. – V.20(7). – P.876-885. doi: 10.1016/j.jval.2017.03.010
38. Nasrallah H.A., Aquila R., Du Y., Stanford A.D., Claxton A., Weiden P.J. Long-term safety and tolerability of aripiprazole lauroxil in patients with schizophrenia. // CNS Spectr. – 2019. – V.24(4). – P.395-403. doi: 10.1017/S1092852918001104
39. Mogwitz S., Buse J., Wolff N., Roessner V. Update on the Pharmacological Treatment of Tics with DopamineModulating Agents. // ACS Chem Neurosci. – 2018. – V.9(4). - P.651-672. doi: 10.1021/acschemneuro.7b00460
40. Zhao Y.J., Lin L., Teng M., Khoo A.L., Soh L.B., et al. Longterm antipsychotic treatment in schizophrenia: systematic review and network meta-analysis of randomized controlled trials. // BJPsych Open. – 2016. – V.2(1). – P.59-66. doi: 10.1192/bjpo.bp.115.002576
41. Maayan N., Quraishi S.N., David A. , Jayaswal A., Eisenbruch M., et al. Fluphenazine decanoate (depot) and enanthate for schizophrenia. // Cochrane Database Syst Rev. – 2015. – V.(2). – P.CD000307. doi: 10.1002/14651858.CD000307.pub2.
, Jayaswal A., Eisenbruch M., et al. Fluphenazine decanoate (depot) and enanthate for schizophrenia. // Cochrane Database Syst Rev. – 2015. – V.(2). – P.CD000307. doi: 10.1002/14651858.CD000307.pub2.
42. Coutinho E., Fenton M., Quraishi S. Zuclopenthixol decanoate for schizophrenia and other serious mental illnesses. // Cochrane Database Syst Rev. - 2000. - V.(2). – P.CD001164. doi: 10.1002/14651858.CD001164
43. Mahapatra J., Quraishi S.N., David A., Sampson S., Adams C.E. Flupenthixol decanoate (depot) for schizophrenia or other similar psychotic disorders. // Cochrane Database Syst Rev. – 2014. – V.(6). – P.CD001470. doi: 10.1002/14651858. CD001470.pub2.
44. Zyprexa [package Insert]. Indianapolis (IN): Eli Lilly and Company; 2018.
45. Fefeu M, De Maricourt P, Cachia A, Hoertel N, Vacheron MN, et al. One-year mirror-image study of the impact of olanzapine long-acting injection on healthcare resource utilization and costs in severe schizophrenia. // Psychiatry Res.