Withdrawal symptoms of viibryd
Vilazodone (Viibryd) | NAMI: National Alliance on Mental Illness
Brand name: Viibryd®
- Tablets: 10 mg, 20 mg, 40 mg
Generic name: vilazodone (vil AZ oh done)
All FDA black box warnings are at the end of this fact sheet. Please review before taking this medication.
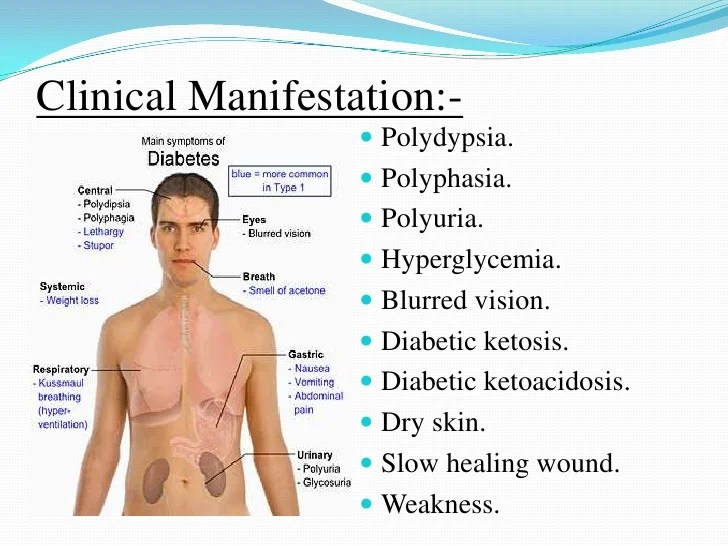
What Is Vilazodone And What Does It Treat?
Vilazodone is an antidepressant medication that works in the brain. It is approved for the treatment of major depressive disorder (MDD).
Symptoms of depression include:
- Depressed mood - feeling sad, empty, or tearful
- Feeling worthless, guilty, hopeless, and helpless
- Loss of interest or pleasure in your usual activities
- Sleep and eat more or less than usual (for most people it is less)
- Low energy, trouble concentrating, or thoughts of death (suicidal thinking)
- Psychomotor agitation (‘nervous energy’)
- Psychomotor retardation (feeling like you are moving and thinking in slow motion)
- Suicidal thoughts or behaviors
What Is The Most Important Information I Should Know About Vilazodone?
Do not stop taking vilazodone, even when you feel better. With input from you, your health care provider will assess how long you will need to take the medicine.
Missing doses of vilazodone may increase your risk for relapse in your symptoms.
Stopping vilazodone abruptly may result in one or more of the following withdrawal symptoms: irritability, nausea, feeling dizzy, vomiting, nightmares, headache, and/or paresthesias (prickling, tingling sensation on the skin).
Depression is also a part of bipolar illness. People with bipolar disorder who take antidepressants may be at risk for "switching" from depression into mania. Symptoms of mania include "high" or irritable mood, very high self-esteem, decreased need for sleep, pressure to keep talking, racing thoughts, being easily distracted, frequently involved in activities with a large risk for bad consequences (for example, excessive buying sprees).
Medical attention should be sought if serotonin syndrome is suspected. Please refer to serious side effects for signs/symptoms.
Are There Specific Concerns About Vilazodone And Pregnancy?
If you are planning on becoming pregnant, notify your health care provider to best manage your medications. People living with MDD who wish to become pregnant face important decisions. Untreated MDD has risks to the fetus, as well as the mother. It is important to discuss the risks and benefits of treatment with your doctor and caregivers.
Caution is advised with breastfeeding since vilazodone does pass into breast milk.
What Should I Discuss With My Health Care Provider Before Taking Vilazodone?
- Symptoms of your condition that bother you the most
- If you have thoughts of suicide or harming yourself
- Medications you have taken in the past for your condition, whether they were effective or caused any adverse effects
- If you experience side effects from your medications, discuss them with your provider. Some side effects may pass with time, but others may require changes in the medication.
- Any other psychiatric or medical problems you have, including a history of bipolar disorder
- All other medications you are currently taking (including over the counter products, herbal and nutritional supplements) and any medication allergies you have
- Other non-medication treatment you are receiving, such as talk therapy or substance abuse treatment. Your provider can explain how these different treatments work with the medication.
- If you are pregnant, plan to become pregnant, or are breastfeeding
- If you drink alcohol or use drugs
How Should I Take Vilazodone?
Vilazodone is usually taken one time per day with food or milk.
Typically patients begin at a low dose of medicine and the dose is increased slowly over several weeks.
The dose usually ranges from 20 mg to 40 mg. Only your health care provider can determine the correct dose for you.
Consider using a calendar, pillbox, alarm clock, or cell phone alert to help you remember to take your medication. You may also ask a family member or friend to remind you or check in with you to be sure you are taking your medication.
You may also ask a family member or friend to remind you or check in with you to be sure you are taking your medication.
What Happens If I Miss A Dose Of Vilazodone?
If you miss a dose of vilazodone, take it as soon as you remember, unless it is closer to the time of your next dose. Discuss this with your health care provider. Do not double your next dose or take more than what is prescribed.
What Should I Avoid While Taking Vilazodone?
Avoid drinking alcohol or using illegal drugs while you are taking antidepressant medications. They may decrease the benefits (e.g., worsen your condition) and increase adverse effects (e.g., sedation) of the medication.
What Happens If I Overdose With Vilazodone?
If an overdose occurs, call your doctor or 911. You may need urgent medical care. You may also contact the poison control center at 1-800-222-1222.
A specific treatment to reverse the effects of vilazodone does not exist.
What Are The Possible Side Effects Of Vilazodone?
Common side effects
Diarrhea, nausea, vomiting, dry mouth, dizziness, insomnia
Rare/serious side effects
Night sweats, decreased appetite, migraine headaches, sleepiness, tremor, blurry vision, dry eyes, abnormal dreams, agitation, restlessness, increased urination, sexual dysfunction, angle closure glaucoma (symptoms of angle closure glaucoma may include eye pain, changes in vision, swelling or redness in or around eye), Serotonin syndrome (symptoms may include shivering, diarrhea, confusion, severe muscle tightness, fever, seizures, and death), palpitations, irregular heartbeat
Vilazodone may increase the risk of bleeding events. Combined use of aspirin, nonsteroidal anti-inflammatory drugs (e.g., ibuprofen, naproxen), warfarin, and other anti-coagulants may increase this risk. This may include gums that bleed more easily, nose bleed, or gastrointestinal bleeding. Some cases have been life threatening.
Combined use of aspirin, nonsteroidal anti-inflammatory drugs (e.g., ibuprofen, naproxen), warfarin, and other anti-coagulants may increase this risk. This may include gums that bleed more easily, nose bleed, or gastrointestinal bleeding. Some cases have been life threatening.
Are There Any Risks For Taking Vilazodone For Long Periods Of Time?
To date, there are no known problems associated with long term use of vilazodone. It is a safe and effective medication when used as directed.
What Other Medications May Interact With Vilazodone?
Vilazodone should not be taken with or within 2 weeks of taking monoamine oxidase inhibitors (MAOIs). These include phenelzine (Nardil®), tranylcypromine (Parnate®), isocarboxazid (Marplan®), rasagiline (Azalect®) and selegiline (Emsam®).
Although rare, there is an increased risk of serotonin syndrome when vilazodone is used with other medications that increase serotonin, such as other antidepressants, migraine medications called “triptans” (e. g., Imitrex®), some pain medications (e.g., tramadol (Ultram®), and the antibiotic linezolid (Zyvox®).
g., Imitrex®), some pain medications (e.g., tramadol (Ultram®), and the antibiotic linezolid (Zyvox®).
The following medications may increase the levels and effects of vilazodone requiring decreased dose of vilazodone:
- Antibiotics, such as clarithromycin (Biaxin®), erythromycin (Ery-Tab®), and telithromycin (Ketek®)
- Antifungals, such as fluconazole (Diflucan®), ketoconazole (Nizoral®), and itraconazole (Sporanox®)
- Blood pressure medications, such as verapamil (Calan®, Covera-HS®, Isoptin SR®) and diltiazem (Cardizem®, Tiazac®)
- HIV medications such as protease inhibitors: indinavir (Crixivan®), ritonavir (Norvir®), saquinavir (Fortovase®, Invirase®), and lopinavir/ritonavir (Kaletra®)
- Nefazodone
The following medications may decrease the levels and effects of vilazodone requiring increased dose of vilazodone: carbamazepine (Tegretol®), oxcarbazepine (Trileptal®), rifampin (Rifadin®), phenytoin (Dilantin®), and phenobarbital
How Long Does It Take For Vilazodone To Work?
Sleep, energy, or appetite may show some improvement within the first 1-2 weeks. Improvement in these physical symptoms can be an important early signal that the medication is working. Depressed mood and lack of interest in activities may need up to 6-8 weeks to fully improve.
Improvement in these physical symptoms can be an important early signal that the medication is working. Depressed mood and lack of interest in activities may need up to 6-8 weeks to fully improve.
Summary of FDA Black Box Warnings
Suicidal thoughts or actions in children and adults
Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications. This risk may persist until significant remission occurs.
risk may persist until significant remission occurs.
In short-term studies, antidepressants increased the risk of suicidality in children, adolescents, and young adults when compared to placebo. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24. Adults age 65 and older taking antidepressants have a decreased risk of suicidality. Patients, their families, and caregivers should be alert to the emergence of anxiety, restlessness, irritability, aggressiveness and insomnia. If these symptoms emerge, they should be reported to the patient’s prescriber or health care professional. All patients being treated with antidepressants for any indication should watch for and notify their health care provider for worsening symptoms, suicidality and unusual changes in behavior, especially during the first few months of treatment.
Adults age 65 and older taking antidepressants have a decreased risk of suicidality. Patients, their families, and caregivers should be alert to the emergence of anxiety, restlessness, irritability, aggressiveness and insomnia. If these symptoms emerge, they should be reported to the patient’s prescriber or health care professional. All patients being treated with antidepressants for any indication should watch for and notify their health care provider for worsening symptoms, suicidality and unusual changes in behavior, especially during the first few months of treatment.
Provided by
(December 2020)
©2020 The College of Psychiatric and Neurologic Pharmacists (CPNP) and the National Alliance on Mental Illness (NAMI). CPNP and NAMI make this document available under the Creative Commons Attribution-No Derivatives 4.0 International License. Last Updated: January 2016.
This information is being provided as a community outreach effort of the College of Psychiatric and Neurologic Pharmacists. This information is for educational and informational purposes only and is not medical advice. This information contains a summary of important points and is not an exhaustive review of information about the medication. Always seek the advice of a physician or other qualified medical professional with any questions you may have regarding medications or medical conditions. Never delay seeking professional medical advice or disregard medical professional advice as a result of any information provided herein. The College of Psychiatric and Neurologic Pharmacists disclaims any and all liability alleged as a result of the information provided herein.
This information is for educational and informational purposes only and is not medical advice. This information contains a summary of important points and is not an exhaustive review of information about the medication. Always seek the advice of a physician or other qualified medical professional with any questions you may have regarding medications or medical conditions. Never delay seeking professional medical advice or disregard medical professional advice as a result of any information provided herein. The College of Psychiatric and Neurologic Pharmacists disclaims any and all liability alleged as a result of the information provided herein.
Viibryd Withdrawal Help | Vilazodone Side Effects, Symptoms
Alternative to Meds News & Blog Articles
Last Updated on December 14, 2022 by Diane Ridaeus
Alternative to Meds Editorial Team
Medically Reviewed by Dr Samuel Lee MD
Table of Contents:
- Video: Viibryd Withdrawal
- Viibryd Withdrawal
- Common Viibryd Withdrawal Symptoms
- Severe and Rare Viibryd Withdrawal Symptoms
- Viibryd Half-life and Withdrawal Timeline
- Can You Stop Taking Viibryd Cold Turkey?
- Tips for Safe, Healthy Viibryd Withdrawal
- About Viibryd Withdrawal Protocols Used at Alternative to Meds Center
- Find Help Now for Viibryd Withdrawal at Alternative to Meds Center
A prescription of Viibryd may not have provided the relief you were seeking and perhaps now is the time to consider Viibryd withdrawal as a positive step along the journey to natural mental health. It is possible to transition to natural alternative treatments without the harsh side effects common to all SSRIs.
It is possible to transition to natural alternative treatments without the harsh side effects common to all SSRIs.
We do not doubt that your suffering is real. But we doubt that you have been correctly treated if you are still suffering. We’ve been there too, and we have a comprehensive non-toxic toolbox to share with you.
Do Your Symptoms Require Viibryd?
Alternative to Meds is a world leader in recovery after prescription drug therapy didn’t meet your expectations. We offer safe, medically monitored Viibryd withdrawal supported by a staff of over 40 amazing practitioners and medical doctors to guide you step by step. Our success rate of over 77% is documented by published evidence and we want you to share in that success. Despite the fact that we have successfully helped over 20,000 clients, we recognize you as a unique individual requiring a tailored treatment program made just for you. We are here to help you reach your mental wellness goals.
We are here to help you reach your mental wellness goals.
Viibryd Withdrawal
Viibryd withdrawal symptoms are common to all antidepressants according to research documents spanning from at least 1959, and continuing on to the present.5-7 Back in the 50s doctors and researchers (and pharmaceutical companies) were looking for something better than electroconvulsive therapy, or even better than nothing. In fact, most drug trials today are still looking for something that is better than nothing (placebo-controlled). Much debate has developed over the decades as to whether the promise of relief to patients has really ever been fulfilled with drug-based therapy. Each new drug was supposed to surpass the last. Perhaps it is time to drop some of the false promises of the past and look forward to real relief through the use of holistic approaches to improved mental wellness. And, with holistic and effective remedies to support Viibryd withdrawal, one can discard the liability of a lifetime of antidepressant side effects and lack of efficacy.
Below you will find some information we hope you will find useful on Viibryd withdrawal including common and more rare adverse Viibryd withdrawal symptoms, strategies to overcome these, and information on how natural and holistic treatments are used at Alternative to Meds Center for a safe and gentle Viibryd withdrawal experience, and recovery of mental wellness without drugs.
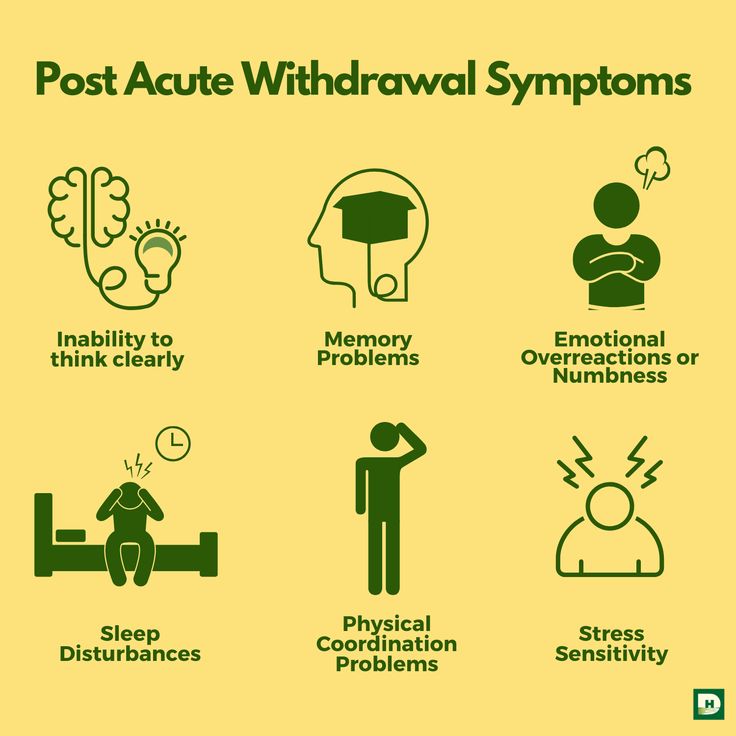
Common Viibryd Withdrawal Symptoms
SPECIAL NOTE RE PREGNANCY: Viibryd is not recommended during late pregnancy due to potential withdrawals in the newborn, as well as heart complications in the infant.1
The most common symptoms of Viibryd withdrawal may resemble those of other SSRIs and involve both physical and psychological symptoms.2,3
Symptoms of Viibryd withdrawal may include:
- Emotional lability: sudden changes in mood, worsening mood, dysphoric mood, crying spells
- Irritability, agitation, anxiety, nervousness
- Flu-like symptoms: headaches, muscle pain, shivers, fatigue, weakness, excessive sweating
- Insomnia, nightmares, unusual or vivid dreams
- Vertigo, dizziness
- Tremors, involuntary jerking motions, ataxia (unsteady or uncoordinated walking)
- Brain zaps or feeling of electric shocks in the head or neck
- Nausea, vomiting
- Abdominal pain
- Constipation, diarrhea
- Paresthesia: burning or prickling skin sensation
- Sensory or visual disturbances, blurred vision
- Sexual dysfunction
- Itching
- Altered taste
- Tinnitus
- Confusion, disorientation
Severe and Rare Viibryd Withdrawal Symptoms
1,4,8-15,20Though relatively rare, there have been severe and even life-threatening reactions to Viibryd withdrawal, especially where the dosage was dropped too quickly or upon abrupt cessation, and where other health conditions were present and untreated. It should be noted that some adverse drug reactions associated with SSRIs may persist after drug discontinuation. Reported rare and severe withdrawal reactions have included the following:
It should be noted that some adverse drug reactions associated with SSRIs may persist after drug discontinuation. Reported rare and severe withdrawal reactions have included the following:
- Suicide, suicidal thinking
- Seizures
- Psychosis, delirium
- Visual and auditory hallucinations
- Mania (severe, lasts at least 7 days) and hypomania (less severe, lasts up to 4 days)
It should be well-noted that published research studies on Viibryd withdrawal are near to non-existent. Studies on other SSRI withdrawal phenomena are much more prevalent and should be considered for the full breadth of the subject. There could be other adverse discontinuation symptoms besides the ones listed above.
Viibryd Half-life and Withdrawal Timeline
The half-life of a medication is how long it takes for the concentration in the system to decrease by half. Viibryd has a half-life estimated at about 25 hours. This may vary from person to person because of individual rates of metabolism, age, and other health considerations. The half-life is typically when withdrawals may begin to manifest, anywhere from a day to several days after the last dose in this case. The medical consensus is that instead of abruptly stopping, decreasing the dose gradually is a recommended strategy to help reduce harsh Viibryd withdrawals.
The half-life is typically when withdrawals may begin to manifest, anywhere from a day to several days after the last dose in this case. The medical consensus is that instead of abruptly stopping, decreasing the dose gradually is a recommended strategy to help reduce harsh Viibryd withdrawals.
There can be no fixed timeline for successfully coming off Viibryd because each individual can have unique responses after lowering the dose. With proper support, preparation, and medical monitoring, the timeline for comfortable Viibryd withdrawal is expected to be 8 weeks but could extend up to one year, depending on the individual factors as noted above.13,18 Clinical research advises that lowering the dose during antidepressant withdrawal should be not only gradual, but that near the end, the doses be incrementally cut even more slowly, and should gradually decrease to levels well below the lowest therapeutic dosage level. Much evidence has shown that a misdiagnosis of relapse is not uncommon when withdrawal symptoms come on too harshly and may be misinterpreted as a relapse. Be informed before you start Viibryd withdrawal so that you have a good grasp on what to expect and you can accurately inform your prescriber what you are experiencing so it will not be misdiagnosed.
Be informed before you start Viibryd withdrawal so that you have a good grasp on what to expect and you can accurately inform your prescriber what you are experiencing so it will not be misdiagnosed.
Can You Stop Taking Viibryd Cold Turkey?
Though some may have been able to abruptly stop Viibryd without many consequences, that would be the exception, and could likely include persons who took Viibryd only for a few days or a few weeks at most or took only very low doses. In any case, a cold turkey approach is not recommended as it can lengthen the process of Viibryd withdrawal, and may encourage misdiagnosis and incorrect treatment measures, as previously cautioned, above.18
The recommended route is to go slow and gradual, and if the taper should still become too harsh for the body to tolerate, reinstating at a higher dosage until stable and then resuming the taper more gradually would be preferable to ease the process.
Tips for Safe, Healthy Viibryd Withdrawal
- Enlist competent medical support before starting Viibryd withdrawal.
 Inpatient treatment is best but if this is not possible, put together a solid support team including a prescriber who is sympathetic to your situation and familiar with antidepressant withdrawal. Let trusted friends and/or family know what your plans are so they can understand your efforts. A spouse or close associate may be able to assist with cooking, cleaning, or other chores. Don’t be afraid to ask for help, even if it is only for some moments of companionship with someone you feel comfortable spending quiet time with. Most people are happy and quite willing to help others given the opportunity to do so.
Inpatient treatment is best but if this is not possible, put together a solid support team including a prescriber who is sympathetic to your situation and familiar with antidepressant withdrawal. Let trusted friends and/or family know what your plans are so they can understand your efforts. A spouse or close associate may be able to assist with cooking, cleaning, or other chores. Don’t be afraid to ask for help, even if it is only for some moments of companionship with someone you feel comfortable spending quiet time with. Most people are happy and quite willing to help others given the opportunity to do so. - You may find it necessary to take time off work or school for a time to allow for a more relaxed schedule, extra rest, outdoor walks, reading, or other mild, relaxing activities, and to keep environmental stress to an absolute minimum during Viibryd withdrawal.
- Stay in close contact with your prescriber if you are not able to enter an inpatient setting, so that minor changes in dosage (which can make major differences) can be well-managed as you go through the process.
If you have attempted Viibryd withdrawal and found the process too difficult to manage on your own, we strongly recommend considering inpatient help. At Alternative to Meds Center, we specialize in the most difficult of cases. The process of Viibryd withdrawal need not be torturous or overly long with the proper help in place. We are here to help.
About Viibryd Withdrawal Protocols Used at Alternative to Meds Center
Protocols used in antidepressant withdrawal have been carefully designed at the center, and offer services that can markedly ease the process, including these examples:
- Holistic, medically monitored slow and gradual medication tapering.
- Orthomolecular medicine
- Neurotoxin Removal
- Neurotransmitter rehabilitation
- IV + NAD therapy
- Counseling services
- Acupuncture
- Massage and spa services
- Equine-assisted therapy
- Nebulized glutathione
- Colon hydrotherapy
We invite you to visit a more complete description of other therapies that can be found on our services overview page where they are described in much greater detail.
Find Help Now for Viibryd Withdrawal at Alternative to Meds Center
At the Alternative to Meds Center, we have been helping thousands of clients for more than 15 years to reach their goals by thoroughly investigating and addressing root causes for symptoms using effective, drug-free therapeutics that can provide authentic relief.
Please contact us for more information for you or your loved one on the very real opportunity to experience recovery of natural mental health through safe Viibryd withdrawal and get the tools for true relief from the symptoms that drug therapy could not resolve.
1 (800) 301-3753 for Viibryd Withdrawal Help
1. FDA label Viibryd (vilazodone hydrochloride) tablets, approval 2011 [cited 2022 May 6]
2. Siwek M, Chrobak AA, Gorostowicz A, Krupa AJ, Dudek D. Withdrawal Symptoms Following Discontinuation of Vortioxetine-Retrospective Chart Review. Pharmaceuticals (Basel). 2021;14(5):451.
Published 2021 May 11. doi:10.3390/ph24050451 [cited 2022 May 6]
3. Haddad P, Anderson I, Recognizing and managing antidepressant discontinuation [published online 2 Jan 2018] [cited 2022 May 6]
4. Dailey MW, Saadabadi A. Mania. [Updated 2021 Aug 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493168/ [cited 2022 May 6]
5. Mann A, MacPherson A, Clinical Experience with Imipramine (G22355) in the Treatment of Depression, Canadian Journal of Psychiatry Vol4, no.1, January 1, 1959 [online] [cited 2022 May 6]
6. Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998 Jul 15;44(2):77-87. doi: 10.1016/s0006-3223(98)00126-7. PMID: 9646889. [cited 2022 May 6]
7. Hengartner MP, Plöderl M. Prophylactic effects or withdrawal reactions? An analysis of time-to-event data from antidepressant relapse prevention trials submitted to the FDA.
Ther Adv Psychopharmacol. 2021 Aug 10;11:20451253211032051. doi: 10.1177/20451253211032051. PMID: 34394912; PMCID: PMC8361519. [cited 2022 May 6]
8. Lane RM. SSRI-induced extrapyramidal side-effects and akathisia: implications for treatment. J Psychopharmacol. 1998;12(2):192-214. doi: 10.1177/026988119801200212. PMID: 9694033. [cited 2022 May 6]
9. Batla A. Dystonia: A review. Neurol India. 2018 Mar-Apr;66(Supplement):S48-S58. doi: 10.4103/0028-3886.226439. PMID: 29503327. [cited 2022 May 6]
10. Brigo F, Erro R, Marangi A, Bhatia K, Tinazzi M. Differentiating drug-induced parkinsonism from Parkinson’s disease: an update on non-motor symptoms and investigations. Parkinsonism Relat Disord. 2014 Aug;20(8):808-14. doi: 10.1016/j.parkreldis.2014.05.011. Epub 2014 Jun 3. PMID: 24935237. [cited 2022 May 6]
11. Friedman JH. Movement disorders induced by psychiatric drugs that do not block dopamine receptors. Parkinsonism Relat Disord. 2020 Oct;79:60-64. doi: 10.1016/j.
parkreldis.2020.08.031. Epub 2020 Aug 26. PMID: 32871538. [cited 2022 May 6]
12. Perahia DG, Kajdasz DK, Desaiah D, Haddad PM. Symptoms following abrupt discontinuation of duloxetine treatment in patients with major depressive disorder. J Affect Disord. 2005 Dec;89(1-3):207-12. doi: 10.1016/j.jad.2005.09.003. Epub 2005 Nov 2. PMID: 16266753. [cited 2022 May 6]
13. Henssler J, Heinz A, Brandt L, Bschor T. Antidepressant Withdrawal and Rebound Phenomena. Dtsch Arztebl Int. 2019;116(20):355-361. doi:10.3238/arztebl.2019.0355 [cited 2022 May 6]
14. Rusconi AC, Carlone C, Muscillo M, Coccanari de’ Fornari MA, Podda L, Piccione M. Sindrome da sospensione di SSRI: incidenza e differenze su tre gruppi di pazienti in trattamento con paroxetina [SSRI discontinuation syndrome: incidence and differences on three groups of patients treated with paroxetine]. Riv Psichiatr. 2009 May-Jun;44(3):169-75. Italian. PMID: 20066803. [cited 2022 May 6]
15. Simeon D, Stein DJ, Hollander E.
Depersonalization disorder and self-injurious behavior. J Clin Psychiatry. 1995;56 Suppl 4:36-9; discussion 40. PMID: 7713864. [cited 2022 May 6]
16. Wolfe RM. Antidepressant withdrawal reactions. Am Fam Physician. 1997 Aug;56(2):455-62. Erratum in: Am Fam Physician 1998 Feb 15;57(4):646. PMID: 9262526. [cited 2022 May 6]
17. Schwasinger-Schmidt TE, Macaluso M. Other Antidepressants. Handb Exp Pharmacol. 2019;250:325-355. doi: 10.1007/164_2018_167. PMID: 30194544. [cited 2022 May 6]
18. Gabriel M, Sharma V. Antidepressant discontinuation syndrome. CMAJ. 2017;189(21):E747. doi:10.1503/cmaj.160991 [cited 2022 May 6]
19. Cruz MP. Vilazodone HCl (Viibryd): A Serotonin Partial Agonist and Reuptake Inhibitor For the Treatment of Major Depressive Disorder. P T. 2012;37(1):28-31. [cited 2022 May 6]
20. Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature.
Psychother Psychosom. 2016;85(5):270-88. doi: 10.1159/000447034. Epub 2016 Aug 11. PMID: 27508501. 9cited 2022 May 6]
Originally Published May 5, 2022 by Diane Ridaeus
This content has been reviewed and approved by a licensed physician.
Dr. Samuel Lee
Dr. Samuel Lee is a board-certified psychiatrist, specializing in a spiritually-based mental health discipline and integrative approaches. He graduated with an MD at Loma Linda University School of Medicine and did a residency in psychiatry at Cedars-Sinai Medical Center and University of Washington School of Medicine in Seattle. He has also been an inpatient adult psychiatrist at Kaweah Delta Mental Health Hospital and the primary attending geriatric psychiatrist at the Auerbach Inpatient Psychiatric Jewish Home Hospital. In addition, he served as the general adult outpatient psychiatrist at Kaiser Permanente. He is board-certified in psychiatry and neurology and has a B.A. Magna Cum Laude in Religion from Pacific Union College. His specialty is in natural healing techniques that promote the body’s innate ability to heal itself.
His specialty is in natural healing techniques that promote the body’s innate ability to heal itself.
Social Profile: LinkedIn
View Bio
Diane Ridaeus
Diane is an avid supporter and researcher of natural mental health strategies. Diane received her medical writing and science communication certification through Stanford University and has published over 3 million words on the topics of holistic health, addiction, recovery, and alternative medicine. She has proudly worked with the Alternative to Meds Center since its inception and is grateful for the opportunity to help the founding members develop this world-class center that has helped so many thousands regain natural mental health.
Medical Disclaimer:
Nothing on this Website is intended to be taken as medical advice. The information provided on the website is intended to encourage, not replace, direct patient-health professional relationships. Always consult with your doctor before altering your medications. Adding nutritional supplements may alter the effect of medication. Any medication changes should be done only after proper evaluation and under medical supervision.
Adding nutritional supplements may alter the effect of medication. Any medication changes should be done only after proper evaluation and under medical supervision.
Our Success Stories
Medication Withdrawal Success Stories
Can you imagine being free from medications, addictive drugs, and alcohol? This is our goal and we are proving it is possible every day!
Read All StoriesView All Videos
Happy Story of Geodon Taper & Withdrawal
“Going through the program at ATMC has genuinely changed my life! I will be forever grateful …” My name...Cannabis-Induced Psychosis
Alternative to Meds Editorial Team Medically Reviewed by Dr Samuel Lee MD Table of Contents: What Is Cannabis-Induced Psychosis?.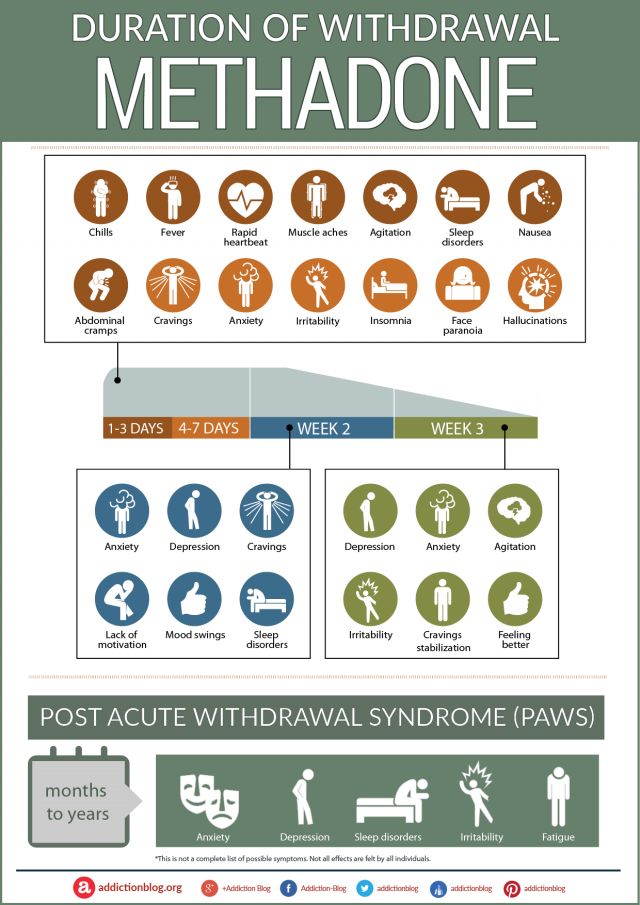 ..
..Successful Withdrawal from Abilify
Prior to becoming a resident at ATMC, I had been on the highest recommended dose of Abilify, as well...Freedom from Antidepressants, Recovery from Mental Breakdown
The hate is gone. The anger is gone. The judgment is gone. I am a new man. 28 Days...Coming to ATMC was Life-Changing
I believe coming to ATMC was a life-changing/turning point experience. When I first came to ATMC I was hopeful,...Cymbalta Withdrawal Success
Today I am home and completely off of cymbalta with minimal effects … thanks to one month at Alternative. ..
..The Sauna Program, Diet Change and Supplementation at ATMC Changed My Life
I came to ATMC on four psych meds: Wellbutrin, Lamictal, Ritalin and Gabapentin. I have been trying to taper...Detox from Anti-Depressants, Opiates and Benzos
I came to ATMC to detox from anti-depressants, opiate and benzos. When I arrived, I was completely hopeless. Had...Life Before ATMC Was Scary And Stressful
I came into ATMC, not on any meds specifically. My doctor gave me Lorazapam to get here but it...
Viibryd Side Effects: What You Need to Know
Introduction
If you have depression, your doctor may suggest Viibryd (vilazodone) as a treatment option for your condition. Information about the possible side effects of this drug can help you decide if it is right for you.
Information about the possible side effects of this drug can help you decide if it is right for you.
Viibryd is a prescription drug used to treat major depressive disorder (MDD) in adults. With MDD, you have an imbalance in the levels of certain chemicals in your brain. Viibryd is an antidepressant that helps correct this chemical imbalance. Over time, this helps reduce symptoms of depression and feel like yourself again. nine0005
Viibryd comes as tablets that you take by mouth once a day. You usually need to take it for several months or longer.
For more information about Viibryd, including usage information, see this detailed product article.
Like all drugs, Viibryd can cause mild or serious side effects. Keep reading to find out more.
What are the most common side effects of Viibryd? nine0003
Some people may experience mild or severe side effects while taking Viibryd. Examples of the most commonly reported side effects of Viibryd include:
- nausea
- diarrhea
- dry mouth
- trouble sleeping
What are the mild side effects of Viibryd?
Examples of mild side effects reported with Viibryd include:
- nausea and vomiting
- diarrhea
- Dry mouth
- stomach Disorders
- Lumber pain
- Headache*
- Dizziness
- Problems with
- Unusual dreams
- Sexual problems, such as erectile dysfunction, decrease in sexual loss or problems with an orgasm
- drowsiness
- fatigue (low energy)
- weight gain or weight loss*
In most cases, these side effects should be temporary. Some of them are easy to manage too. But if you have any symptoms that continue or bother you, talk to your doctor or pharmacist. And don't stop using Viibryd unless your doctor recommends it. nine0005
Some of them are easy to manage too. But if you have any symptoms that continue or bother you, talk to your doctor or pharmacist. And don't stop using Viibryd unless your doctor recommends it. nine0005
Viibryd may cause mild side effects other than those listed above. See the Viibryd Medication Guide for details.
After the Food and Drug Administration (FDA) approves a drug, it monitors and analyzes the drug's side effects. If you would like to notify the FDA of a side effect you have had with Viibryd, visit MedWatch.
What are the serious side effects of Viibryd?
Serious side effects of Viibryd are rare but may occur. Serious side effects reported with Viibryd include:
- bleeding more than usual
- mania or hypomania (periods of high energy or racing thoughts)
- seizures
- angle-closure glaucoma (sudden increase in intraocular pressure)
- hyponatraemia (low sodium102 and suicidal actions) 909 *
- serotonin syndrome†
- allergic reaction†‡
If you develop serious side effects while taking Viibryd, call your doctor right away. If the side effects seem life-threatening or if you think you need emergency medical attention, call 9 right away11 or your local emergency number.
If the side effects seem life-threatening or if you think you need emergency medical attention, call 9 right away11 or your local emergency number.
Suicide Prevention
If you think someone is at immediate risk of harming themselves or harming another person:
- Call 911 or your local emergency number.
- Stay with the person until help arrives.
- Remove all weapons, knives, medicines, and other items that could cause harm. nine0020
- Listen, but don't judge, argue, threaten or yell.
If you or someone you know is thinking about suicide, get help from a crisis or suicide prevention hotline. Call the National Suicide Prevention Hotline at 800-273-8255.
Frequently Asked Questions About Viibryd Side Effects
Here are answers to some frequently asked questions about Viibryd side effects. nine0005
When do the side effects of Viibryd usually disappear?
Most mild side effects of Viibryd disappear within a few days to a couple of weeks. This is because your body gets used to the drug. But some, such as sexual problems, may last longer. If you have side effects that do not go away, talk to your doctor or pharmacist.
This is because your body gets used to the drug. But some, such as sexual problems, may last longer. If you have side effects that do not go away, talk to your doctor or pharmacist.
If you have serious side effects with Viibryd, they usually improve quickly with treatment. nine0005
Is anger a side effect of Viibryd?
Maybe. Irritability has been reported in some people taking Viibryd and this may manifest as anger.
Mood changes such as anger, irritability or aggression can also be warning signs of worsening depression and suicidal thoughts. In some people, depression may worsen despite taking Viibrid, and this may lead to suicidal thoughts.
But in young people (aged 18 to 24 years), Viibryd may also increase the risk of suicidal thoughts and actions. To learn more about this side effect, see the "Explanation of side effects" section below. nine0005
If you have mood changes such as anger while taking Viibryd, talk to your doctor. They can help determine if it is a side effect of the medication.
Please note that mood changes such as anger are possible withdrawal side effects that may occur after Viibryd treatment is stopped. These side effects can also occasionally occur after missing a dose of Viibryd. See the questions below for more on this.
Will stopping my Viibryd treatment cause withdrawal side effects?
Yes, stopping treatment with Viibrid can sometimes cause withdrawal side effects. This is also called the withdrawal syndrome.
Examples of side effects of withdrawal may include:
- Headache
- nausea
- sweating
- mood swings
- feeling irritated, restless or excited
- confusion
- 0020
- pins and needles or electric shock sensations
- dizziness
- tremors
- trouble sleeping
You are more likely to get withdrawal side effects if you suddenly stop taking Viibryd. If you and your doctor agree that you should stop taking Viibrid, your doctor will explain how to do this gradually. This should help avoid the cancellation effect.
This should help avoid the cancellation effect.
Will I experience any side effects if I miss a dose of Viibryd? nine0115
You are unlikely to have side effects if you miss one dose of Viibryd. But skipping more than one dose can sometimes cause withdrawal side effects. These are the side effects that may occur if you suddenly stop taking Viibryd. See the question above for more on this.
If you have withdrawal side effects after missing a dose of Viibryd, they should get better after the next dose. Do not take additional doses to make up for a missed dose or to alleviate withdrawal side effects. Taking too much Viibryd can cause serious side effects. nine0005
Do the side effects of Viibryd vary with tablet strength (10mg, 20mg or 40mg)?
Not really. In studies with Viibryd, side effects were very similar between the 20 milligram (mg) dose and the 40 mg dose. (The 10 mg tablet is usually taken only during the first week of treatment.)
Your risk of side effects with Viibryd is likely to depend on factors other than dose. For example, certain medications or conditions may increase the risk of side effects more than taking higher doses. nine0005
For example, certain medications or conditions may increase the risk of side effects more than taking higher doses. nine0005
If you are concerned about the risk of side effects from higher doses of Viibryd, talk to your doctor.
Side effects explained
Learn more about some of the side effects Viibryd may cause.
Weight gain or weight loss
Some people may gain or lose weight while taking Viibryd, but this is not common.
In studies with Viibryd, several people experienced weight gain or increased appetite. Increased appetite can lead to weight gain over time. According to the manufacturer, the average weight gained with Viibryd was between 0.35 and 1.3 pounds (lbs). nine0005
Viibryd studies did not specifically report weight loss. But the medicine usually causes diarrhea, nausea, and vomiting. And these digestive side effects can lead to weight loss.
Please note that depression often affects appetite and eating habits, which can lead to weight changes. As your depression eases after treatment with Viibryd, your appetite and eating habits will likely return to what you usually do. And this can lead to you gaining or losing weight. nine0005
As your depression eases after treatment with Viibryd, your appetite and eating habits will likely return to what you usually do. And this can lead to you gaining or losing weight. nine0005
What can help
If you're worried about gaining or losing weight with Viibryd, talk to your doctor. They can suggest ways to help you achieve or maintain a moderate body weight.
Headache
Viibryd may occasionally cause headache. Headaches caused by Viibryd are usually mild. They usually go away with time as your body gets used to the medication.
But sometimes a headache can be a symptom of a more serious side effect of Viibrid, such as hyponatremia (low sodium in the blood). And if you suddenly have a severe headache, it may be a symptom of angle-closure glaucoma (a sudden increase in intraocular pressure). This is another serious side effect of Viibryd. nine0005
What can help
If you get a headache while taking Viibrid, it usually goes away on its own. But it can help to lie down, rest, and turn off the bright lights.
But it can help to lie down, rest, and turn off the bright lights.
If you have a severe headache, you can take an over-the-counter (OTC) pain reliever that contains acetaminophen, such as Tylenol, to relieve it. It's best to avoid products containing aspirin, ibuprofen (Advil, Motrin), or naproxen (Aleve). This may increase the risk of bleeding, which is a rare but serious side effect of Viibryd. Ask your pharmacist to recommend a product that is safe for you. nine0005
If you have a headache that doesn't go away or is very bad, see your doctor.
Serotonin Syndrome
Viibryd can sometimes cause a rare but serious side effect called serotonin syndrome. This leads to increased levels of the chemical serotonin in the body. This side effect can be life-threatening.
Serotonin syndrome can cause symptoms such as:
- rapid heartbeat
- sweating
- Redness (temporary heat, redness or increased skin color)
- Dizziness
- Nausea or vomiting
- DIOLS
- Agitation
- Hallucinations (see or hear unrealistic things)
- TREATIONS or twisting
- Hard muscles
- Loss of cord
- seizures
Serotonin syndrome rarely develops with Viibryd if you take it alone. But taking Viibrid with other drugs that increase serotonin levels in the body can increase the risk of this side effect. Examples of such drugs include:
But taking Viibrid with other drugs that increase serotonin levels in the body can increase the risk of this side effect. Examples of such drugs include:
- Some other antidepressants, such as:
- parksetin (Paxil, Peksva)
- CERTRALIN (ZolOFT)
- Citalopram (CELEXA)
- amitriptyline
- imipramine (Tofranil)
- lithium (Litobid), a drug for treating bipolar disorder and depression 9001
- skin rash
- itching
- redness (temporary warmth, redness, or increased skin color)
- swelling under the skin, usually on the eyelids, lips, hands or feet
- swelling of the mouth, tongue, or throat that makes breathing difficult
- worsening depression or anxiety
- feeling anxious or agitated
- anger, irritability, or aggression
- thinking of harming or killing yourself
- attempting suicide
- other unusual or sudden changes in your mood, thoughts, or actions
- what dose of the drug you were taking when you had the side effect
- how soon after starting this dose you had the side effect
- what were your symptoms due to for a side effect
- how it affected your daily activities
- what other medications you also took
- any other information you think is important
- Do my other medicines increase the risk of side effects when taking Viibryd?
- Am I more likely to get side effects with Viibryd than with other antidepressants? nine0020
- Is there anything I can do to reduce the risk of side effects while taking Viibryd?
- Do I need to stop taking Viibrid if I have surgery?
- De Diego-Adeliño J, Crespo JM, Mora F, et al. Vortioxetine in major depressive disorder: from mechanisms of action to clinical studies. An updated review. Expert Opin Drug Saf. 2022;21(5):673-690. https://doi.org/10.1080/14740338.2022.2019705
- Chen G, Højer AM, Areberg J, et al.
 Vortioxetine: clinical pharmacokinetics and other interactions. Clin Pharmacokinet. 2018;57(6):673-686.
Vortioxetine: clinical pharmacokinetics and other interactions. Clin Pharmacokinet. 2018;57(6):673-686. - Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015;145:43-57.
- Nackenoff AG, Simmler LD, Baganz NL, et al. Serotonin transporter-independent actions of the antidepressant vortioxetine as revealed using the SERT Met172 mouse. ACS Chem Neurosci. 2017;8(5):1092-1100.
- Li Y, Raaby KF, Sánchez C, et al. Serotonergic receptor mechanisms underlying antidepressant-like action in the progesterone withdrawal model of hormonally induced depression in rats. Behav Brain Res. 2013;256:520-528.
- Hlavacova N, Li Y, Pehrson A, et al. Effects of vortioxetine on biomarkers associated with glutamatergic activity in an SSRI insensitive model of depression in female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:332-338.
- Riga MS, Sánchez C, Celada P, et al.
 Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: focus on glutamatergic and GABAergic neurotransmission. neuropharmacology. 2016;108:73-81.
Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: focus on glutamatergic and GABAergic neurotransmission. neuropharmacology. 2016;108:73-81. - Du Jardin KG, Müller HK, Sanchez C, et al. A single dose of vortioxetine, but not ketamine or fluoxetine, increases plasticity-related gene expression in the rat frontal cortex. Eur J Pharmacol. 2016;786:29-35.
- Bora E, Harrison BJ, Yücel M, et al. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43(10):2017-2026. nine0020
- Etkin A, Patenaude B, Song YJ, et al. A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. neuropsychopharmacology. 2015;40(6):1332-1342.
- Bennabi D, Haffen E, Van Waes V. Vortioxetine for cognitive enhancement in major depression: from animal models to clinical research. Front Psychiatry. 2019;10:771.
- Zuena AR, Maftei D, Alemà GS, et al. Multimodal antidepressant vortioxetine causes analgesia in a mouse model of chronic neuropathic pain.
 Mol Pain. 2018;14:1744806918808987.
Mol Pain. 2018;14:1744806918808987. - Fourrier C, Sampson E, Mills NT, et al. Anti-inflammatory treatment of depression: study protocol for a randomized controlled trial of vortioxetine augmented with celecoxib or placebo. trials. 2018;19(1):447.
- Thase ME, Mahableshwarkar AR, Dragheim M, et al. A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol. 2016;26(6):979-993.
- Citrome L. Vortioxetine for major depressive disorder: an indirect comparison with duloxetine, escitalopram, levomilnacipran, sertraline, venlafaxine, and vilazodone, using number needed to treat, number needed to harm, and likelihood to be helped or harmed. JAffect Discord. 2016;196:225-233.
- Thase ME, Danchenko N, Brignone M, et al. Comparative evaluation of vortioxetine as a switch therapy in patients with major depressive disorder. Eur Neuropsychopharmacol. 2017;27(8):773-781.
- Brignone M, Diamand F, Painchault C, et al.
 Efficacy and tolerability of switching therapy to vortioxetine versus other antidepressants in patients with major depressive disorder. Curr Med Res Opin. 2016;32(2):351-366.
Efficacy and tolerability of switching therapy to vortioxetine versus other antidepressants in patients with major depressive disorder. Curr Med Res Opin. 2016;32(2):351-366. - Baune BT, Brignone M, Larsen KG. A network meta-analysis comparing the effects of various antidepressant classes on the digit symbol substitution Test (DSST) as a measure of cognitive dysfunction in patients with major depressive disorder. Int J Neuropsychopharmacol. 2018;21(2):97-107.
- Lenze EJ, Stevens A, Waring JD, et al. Augmenting computerized cognitive training with vortioxetine for age-related cognitive decline: a randomized controlled trial. Am J Psychiatry. 2020;177(6):548-555.
- Cumbo E, Cumbo S, Torregrossa S, et al. Treatment Effects of Vortioxetine on Cognitive Functions in Mild Alzheimer's Disease Patients with Depressive Symptoms: a 12 Month, Open-Label, Observational Study. J Prev Alzheimers Dis. 2019;6(3):192-197.
- Strelnikova I.A., Svetkina A.A., Minina Yu.D.
:max_bytes(150000):strip_icc()/prozac-withdrawal-symptoms-timeline-and-treatment-4766892_final_edit-ddf616fc766d4651afa6b5d804721eea.png) Experience in the use of vortioxetine in the treatment of post-stroke depression. Neurology, neuropsychiatry, psychosomatics. 2020;12(1):45-49.
Experience in the use of vortioxetine in the treatment of post-stroke depression. Neurology, neuropsychiatry, psychosomatics. 2020;12(1):45-49. - Jacobson W, Zhong W, Nomikos GG, et al. Effects of vortioxetine on functional capacity across different levels of functional impairment in patients with major depressive disorder: a University of California, San Diego Performance-based Skills Assessment (UPSA) analysis. Curr Med Res Opin. 2020;36(1):117-124. nine0020
- McIntyre RS, Harrison J, Loft H, et al., The effects of vortioxetine on cognitive function in patients with major depressive disorder: a meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(10):yw055.
- Chokka P, Bougie J, Proulx J, et al. Long-term functioning outcomes are predicted by cognitive symptoms in working patients with major depressive disorder treated with vortioxetine: results from the AtWoRC study. CNS Spectr. 2019;24(6):616-627. nine0020
- Florea I, Loft H, Danchenko N, et al.
 The effect of vortioxetine on overall patient functioning in patients with major depressive disorder. Brainbehav. 2017;7(3):e00622.
The effect of vortioxetine on overall patient functioning in patients with major depressive disorder. Brainbehav. 2017;7(3):e00622. - Cao B, Park C, Subramaniapillai M, et al. The effect of vortioxetine on anhedonia in patients with major depressive disorder. Front Psychiatry. 2019;10:17.
- Fagiolini A, Florea I, Loft H, et al. Effectiveness of vortioxetine on emotional blunting in patients with major depressive disorder with inadequate response to SSRI/SNRI treatment. JAffect Discord. 2021;283:472-479.
- Cronquist MC, Florea I, Lindsten A, et al. Efficacy of vortioxetine on the physical symptoms of major depressive disorder. J Psychopharmacol. 2018;32(10):1086-1097.
- Liguori C, Ferini-Strambi L, Izzi F, et al. Preliminary evidence that vortioxetine may improve sleep quality in depressed patients with insomnia: a retrospective questionnaire analysis. Br J Clin Pharmacol. 2019;85(1):240-244.
- Mahableshwarkar AR, Affinito J, Reines EH, et al.
 Suicidal ideation and behavior in adults with major depressive disorder treated with vortioxetine: post hoc pooled analyses of randomized, placebo-controlled, short-term and open-label, long-term extension trials. CNS Spectr. 2019;14:1-11.
Suicidal ideation and behavior in adults with major depressive disorder treated with vortioxetine: post hoc pooled analyses of randomized, placebo-controlled, short-term and open-label, long-term extension trials. CNS Spectr. 2019;14:1-11. - Baldwin DS, Florea I, Jacobsen PL, et al. A meta-analysis of the efficacy of vortioxetine in patients with major depressive disorder (MDD) and high levels of anxiety symptoms. JAffect Discord. 2016;206:140-150.
- Di Nicola M, Pepe M, Panaccione I, et al. Effect of vortioxetine in subjects with major depressive and alcohol use disorders: a 6-month retrospective analysis. CNS Spectr. 2022;27(1):73-81. https://doi.org/10.1017/S109285292000173X
- Nomikos GG, Tomori D, Zhong W, et al. Efficacy, safety, and tolerability of vortioxetine for the treatment of major depressive disorder in patients aged 55 years or old. CNS Spectr. 2017;22(4):348-362. nine0020
- Freeman MP, Cheng LJ, Moustafa D, et al. Vortioxetine for major depressive disorder, vasomotor, and cognitive symptoms associated with the menopausal transition.

If you have symptoms of serotonin syndrome while you are taking Viibryd, call your doctor right away. But if your symptoms are severe or seem life-threatening, call 911 or your local emergency number.
Serotonin syndrome usually goes away as soon as you stop taking the drug that is causing the problem. But your doctor may also prescribe other medications to help relieve symptoms.
Allergic reaction
Like most medicines, Viybrid may cause an allergic reaction in some people. But it is not clear if this side effect occurred in studies. nine0005
But it is not clear if this side effect occurred in studies. nine0005
Symptoms may be mild or severe and may include:
What may help
If you have mild symptoms of an allergic reaction, such as a mild rash, call your doctor right away. To manage your symptoms, they may suggest an over-the-counter antihistamine you take by mouth, such as Benadryl (diphenhydramine). Or they may recommend a product you apply to your skin, such as a hydrocortisone cream. nine0005
If your doctor confirms that you had a mild allergic reaction to Viibryd, they will decide whether you should continue using it.
If you have symptoms of a severe allergic reaction, such as swelling or difficulty breathing, call 911 or your local emergency number right away. These symptoms can be life threatening and require immediate medical attention.
These symptoms can be life threatening and require immediate medical attention.
If your doctor confirms that you have had a serious allergic reaction to Viibryd, he may ask you to switch to another treatment. nine0005
Suicidal ideation and behavior in children and young people
Like all antidepressants, Viibryd has a boxed warning for the risk of suicidal ideation and behavior in children and young people (aged 18 to 24 years). The boxed warning is the most serious warning from the Food and Drug Administration (FDA). It warns doctors and patients about drug side effects that can be dangerous.
Please note that Viibryd is not FDA approved for use by persons under 18 years of age. nine0005
The presence of depression increases the risk of suicidal thoughts and actions. But for younger people, studies show that taking antidepressants may increase this risk early in treatment. The risk is higher during the first few months of treatment and after any dosage changes.
Antidepressants do not increase the risk of suicidal thoughts and actions in older people with depression. In fact, research shows that antidepressants reduce the risk of suicidal thoughts and actions in adults aged 25 and over. nine0005
Possible warning signs of suicidal thoughts and actions may include:
What can help
While taking Viibryd, it is important to be aware of possible warning signs of suicidal thoughts or behavior. It is also helpful to talk about it with friends, family, or caregivers. They can monitor changes in your behavior. If you have any of the warning signs listed above, be sure to contact your doctor immediately.
It is also important that you continue to take Viibryd regularly every day, even if it doesn't seem to work at first. It may take 2 to 4 weeks for Viibryd to start working. And it may take several months or more before your depression eases. nine0005
It may take 2 to 4 weeks for Viibryd to start working. And it may take several months or more before your depression eases. nine0005
If your doctor thinks that Viibryd may make your depression worse, he may recommend switching to a different antidepressant. But this should only be done under close supervision. You should not stop taking Viibrid or change the dosage on your own.
Talk to your doctor if you have questions or concerns about suicidal thoughts or behavior while you are taking Viibryd.
Tracking side effects
While on Viibryd, consider keeping a record of any side effects you experience. You can then share this information with your doctor. This is especially helpful when you first start taking new medications or using a combination of treatments. nine0005
Your side effect notes may include things like:
Taking notes and sharing them with your doctor will help him learn more about how Viibryd is affecting you.
And your doctor can use this information to adjust your treatment plan if necessary.
Warnings for Viibryd
Viibryd comes with several warnings.
Boxed Warning: Suicidal Thoughts and Behaviors in Children and Young Adults
Viybrid has a boxed warning for the risk of suicidal thoughts and behaviors in children and young adults (aged 18 to 24 years). The boxed warning is the most serious warning from the Food and Drug Administration (FDA). It warns doctors and patients about drug side effects that can be dangerous. nine0005
It is important to note that Viibryd is not FDA approved for use by anyone under the age of 18.
See the "Explanation of Side Effects" section above for more information.
Other warnings
Viibryd may not be right for you if you have certain medical conditions or other health conditions. Talk to your doctor about your medical history before taking Viibryd. The list below includes factors to consider.
Allergic reaction. If you have had an allergic reaction to Viibryd or any of its ingredients, you should not take Viibryd. Ask your doctor which other medicines are best for you.
Bleeding problems. Viibrid may make you bleed more easily than usual. If you have any bleeding problems, talk to your doctor about whether it is safe for you to take Viibryd.
History of bipolar disorder, mania or hypomania. nine0093 If you or a close family member have had these mental health problems in the past, Viibryd may not be safe for you. The drug may increase the risk of a manic episode. Before you start Viibryd, talk to your doctor about any mental health issues that have affected you and your family.
Epilepsy or other convulsive conditions. Viibryd may increase the risk of seizures. If you have had seizures in the past, talk to your doctor about whether it is safe for you to take Viibryd. nine0005
Low sodium. Viibrid can sometimes cause hyponatremia (low sodium in the blood). If you already have low sodium levels, Viibryd may make things worse. This side effect is more common in people 65 years of age or older and in people who take diuretics. If any of the factors apply to you, ask your doctor if it is safe for you to take Viibryd. If your sodium level becomes too low during your Viibryd treatment, you may need to stop taking the drug. nine0005
Viibrid can sometimes cause hyponatremia (low sodium in the blood). If you already have low sodium levels, Viibryd may make things worse. This side effect is more common in people 65 years of age or older and in people who take diuretics. If any of the factors apply to you, ask your doctor if it is safe for you to take Viibryd. If your sodium level becomes too low during your Viibryd treatment, you may need to stop taking the drug. nine0005
Alcohol and Viibryd
Alcohol may increase some of the side effects of Viibryd. For example, it can increase dizziness, drowsiness, nausea, and diarrhea. As a result, you should avoid drinking alcohol while taking Viibryd.
Alcohol can also worsen symptoms of depression.
If you are concerned about not drinking alcohol while taking Viibryd, talk to your doctor.
Pregnancy and lactation while taking Viibrid
It is not known if Viibryd is safe to take while pregnant or breastfeeding.
If you are pregnant or breastfeeding, or planning to become pregnant or breastfeeding, talk to your doctor about the risks and benefits of taking Viibryd.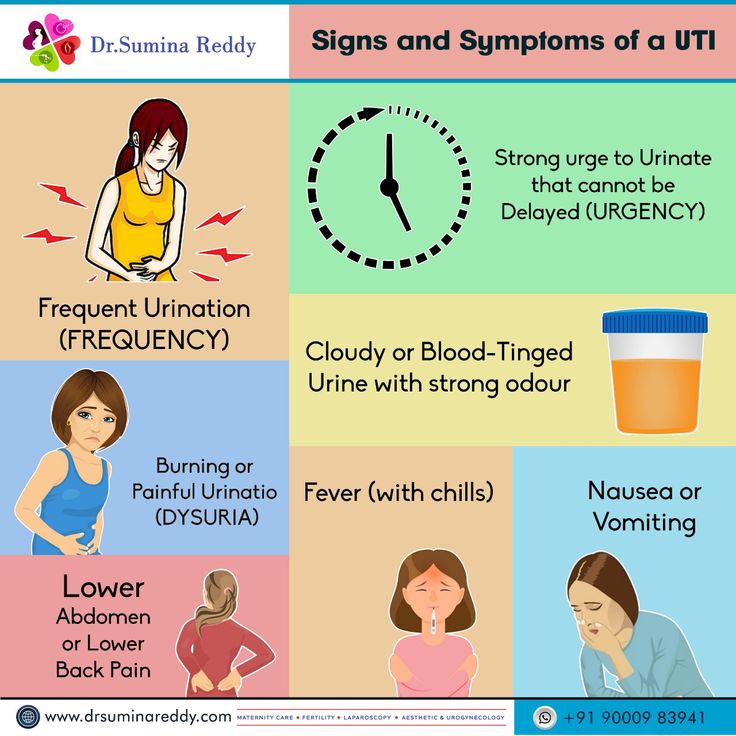
What to ask your doctor
Viibryd is an effective depression medication and most people can take it without problems. It does have some common side effects, but they are usually mild and tend to lessen as your body gets used to the medication. Some serious side effects are also possible, but they are rare. nine0005
Your doctor can give you more information about the potential for side effects while taking Viibryd. And they can help you decide if this medicine is a good treatment option for your condition.
Here are some examples of questions you can ask your doctor:
To read the personal stories of others who have successfully managed their condition, consider subscribing to Drink-Drink's Depression Newsletter.
Ask a pharmacist
Q:
What should I do if Viibrid is preventing me from sleeping?
Anonymous
A:
Mild side effects of Viibryd, such as trouble sleeping, usually resolve within a few days to a couple of weeks. This is because your body gets used to the drug.
But trouble sleeping for long periods of time can make your depression worse. If you are still having trouble sleeping after a few weeks, try taking your daily dose of Viibryd in the morning. Also talk to your doctor. They may suggest other ways to improve sleep or recommend lowering the dosage of the drug. nine0005
Neil Patel, PharmD Answers represent the opinions of our medical experts. All content is for informational purposes only and should not be considered medical advice.
Registration data: Drink-Drink has made every effort to ensure that all information is accurate, complete and up to date. However, this article should not be used as a substitute for the knowledge and experience of a licensed healthcare professional. You should always check with your doctor or other healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or side effects. The absence of warnings or other information for a given medicinal product does not mean that the drug or combination of drugs is safe, effective, or suitable for all patients or for all specific uses. nine0005
However, this article should not be used as a substitute for the knowledge and experience of a licensed healthcare professional. You should always check with your doctor or other healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or side effects. The absence of warnings or other information for a given medicinal product does not mean that the drug or combination of drugs is safe, effective, or suitable for all patients or for all specific uses. nine0005
from mechanisms of action to the clinic
Vortioxetine: from mechanisms of action to the clinic Website of the publishing house "Media Sfera"
contains materials intended exclusively for healthcare professionals. By closing this message, you confirm that you are a registered medical professional or student of a medical educational institution.
Petrova N.N.
St. Petersburg State University
Mukhin A.A.
Company H. Lundbeck - Department of Medicine of the Russian division
Vortioxetine: from mechanisms of action to the clinic
Authors:
Petrova N.N., Mukhin A.A.
More about the authors
Magazine: Journal of Neurology and Psychiatry. S.S. Korsakov. Special issues. 2022;122(6‑2): 84‑90
DOI: 10.17116/jnevro202212206284 nine0005
How to quote:
Petrova N.N., Mukhin A.A. Vortioxetine: from mechanisms of action to the clinic. Journal of Neurology and Psychiatry. S.S. Korsakov. Special issues. 2022;122(6‑2):84‑90.
Petrova NN, Mukhin AA. Mechanisms of action and clinical effects of vortioxetine. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova. nine0585 2022;122(6‑2):84‑90. (In Russ.).
https://doi.org/10.17116/jnevro202212206284
Close metadata
The publication is devoted to the analysis of a large-scale systematic review of current and practice-oriented studies of the use of vortioxetine in depression for the period up to 2020. The mechanisms of multimodal action, issues of efficacy and safety of the drug, including the direct procognitive effect, effects on anhedonia, anxiety, sleep disorders are highlighted , analgesic and anti-inflammatory action. The increase in the effectiveness of vortioxetine with an increase in the dose to 20 mg / day is emphasized. Promising directions of antidepressant research are outlined. nine0005
The mechanisms of multimodal action, issues of efficacy and safety of the drug, including the direct procognitive effect, effects on anhedonia, anxiety, sleep disorders are highlighted , analgesic and anti-inflammatory action. The increase in the effectiveness of vortioxetine with an increase in the dose to 20 mg / day is emphasized. Promising directions of antidepressant research are outlined. nine0005
Keywords:
vortioxetine
mechanisms of action
clinical efficacy
portability
Authors:
Petrova N.N.
St. Petersburg State University
Mukhin A.A.
Company H. Lundbeck - Department of Medicine of the Russian division
References: