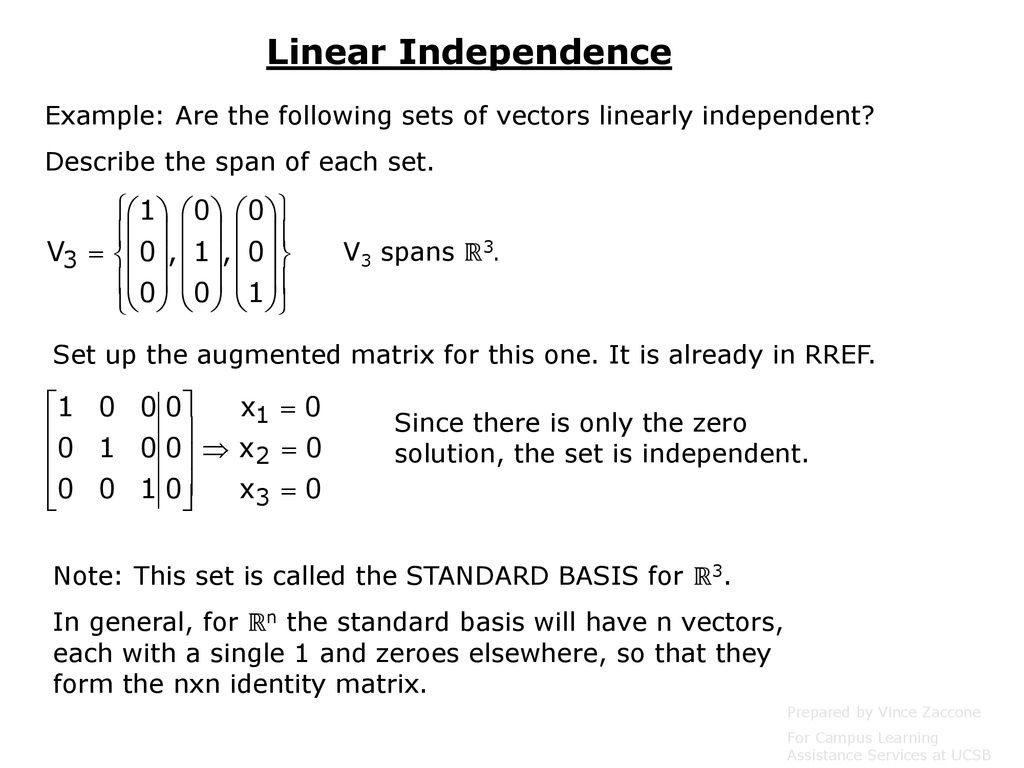
Is lamotrigine the same as lamictal
Generic lamotrigine versus brand-name Lamictal bioequivalence in patients with epilepsy: A field test of the FDA bioequivalence standard
. 2015 Sep;56(9):1415-24.
doi: 10.1111/epi.13095. Epub 2015 Jul 23.
Tricia Y Ting 1 , Wenlei Jiang 2 , Robert Lionberger 2 , Jessica Wong 3 , Jace W Jones 3 , Maureen A Kane 3 , Allan Krumholz 1 , Robert Temple 2 , James E Polli 3
Affiliations
Affiliations
- 1 Department of Neurology, University of Maryland, Baltimore, Maryland, U.
S.A.
- 2 Food and Drug Administration, White Oak, Maryland, U.S.A.
- 3 Department of Pharmaceutical Sciences, University of Maryland, Baltimore, Maryland, U.S.A.
- PMID: 26201987
- DOI: 10.1111/epi.13095
Free article
Tricia Y Ting et al. Epilepsia. 2015 Sep.
Free article
. 2015 Sep;56(9):1415-24.
doi: 10. 1111/epi.13095. Epub 2015 Jul 23.
1111/epi.13095. Epub 2015 Jul 23.
Authors
Tricia Y Ting 1 , Wenlei Jiang 2 , Robert Lionberger 2 , Jessica Wong 3 , Jace W Jones 3 , Maureen A Kane 3 , Allan Krumholz 1 , Robert Temple 2 , James E Polli 3
Affiliations
- 1 Department of Neurology, University of Maryland, Baltimore, Maryland, U.S.A.
- 2 Food and Drug Administration, White Oak, Maryland, U.
 S.A.
S.A. - 3 Department of Pharmaceutical Sciences, University of Maryland, Baltimore, Maryland, U.S.A.
- PMID: 26201987
- DOI: 10.1111/epi.13095
Abstract
Objective: To test the current U.S. Food and Drug Administration (FDA) bioequivalence standard in a comparison of generic and brand-name drug pharmacokinetic (PK) performance in "generic-brittle" patients with epilepsy under clinical use conditions.
Methods: This randomized, double-blind, multiple-dose, steady-state, fully replicated bioequivalence study compared generic lamotrigine to brand-name Lamictal in "generic-brittle" patients with epilepsy (n = 34) who were already taking lamotrigine.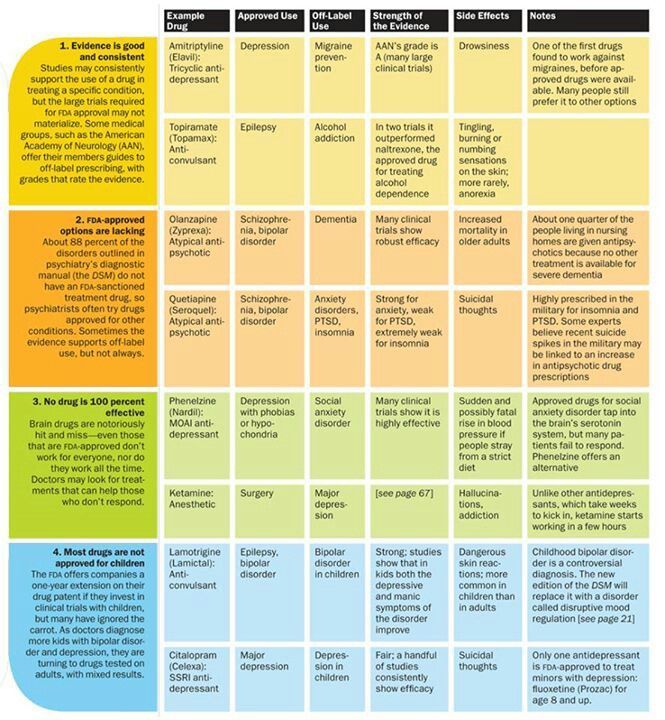 Patients were repeatedly switched between masked Lamictal and generic lamotrigine. Intensive PK blood sampling at the end of each 2-week treatment period yielded two 12-h PK profiles for brand-name and generic forms for each patient. Steady-state area under the curve (AUC), peak plasma concentration (Cmax ), and minimum plasma concentration (Cmin ) data were subjected to conventional average bioequivalence (ABE) analysis, reference-scaled ABE analysis, and within-subject variability (WSV) comparisons. In addition, generic-versus-brand comparisons in individual patients were performed. Secondary clinical outcomes included seizure frequency and adverse events.
Patients were repeatedly switched between masked Lamictal and generic lamotrigine. Intensive PK blood sampling at the end of each 2-week treatment period yielded two 12-h PK profiles for brand-name and generic forms for each patient. Steady-state area under the curve (AUC), peak plasma concentration (Cmax ), and minimum plasma concentration (Cmin ) data were subjected to conventional average bioequivalence (ABE) analysis, reference-scaled ABE analysis, and within-subject variability (WSV) comparisons. In addition, generic-versus-brand comparisons in individual patients were performed. Secondary clinical outcomes included seizure frequency and adverse events.
Results: Generic demonstrated bioequivalence to brand. The 90% confidence intervals of the mean for steady-state AUC, Cmax , and Cmin for generic-versus-brand were 97.2-101.6%, 98.8-104.5%, and 93.4-101.0%, respectively. The WSV of generic and brand were also similar. Individual patient PK ratios for generic-versus-brand were similar but not identical, in part because brand-versus-brand profiles were not identical, even though subjects were rechallenged with the same product. Few subjects had seizure exacerbations or tolerability issues with product switching. One subject, however, reported 267 focal motor seizures, primarily on generic, although his brand and generic PK profiles were practically identical.
Individual patient PK ratios for generic-versus-brand were similar but not identical, in part because brand-versus-brand profiles were not identical, even though subjects were rechallenged with the same product. Few subjects had seizure exacerbations or tolerability issues with product switching. One subject, however, reported 267 focal motor seizures, primarily on generic, although his brand and generic PK profiles were practically identical.
Significance: Some neurologists question whether bioequivalence in healthy volunteers ensures therapeutic equivalence of brand and generic antiepileptic drugs in patients with epilepsy, who may be at increased risk for problems with brand-to-generic switching. Bioequivalence results in "generic-brittle" patients with epilepsy under clinical conditions support the soundness of the FDA bioequivalence standards. Adverse events on generic were not related to the small, allowable PK differences between generic and brand.
Keywords: Bioequivalence; Generic-brittle; Lamotrigine; Narrow therapeutic index; Switchability.
Wiley Periodicals, Inc. © 2015 International League Against Epilepsy.
Similar articles
-
Bioequivalence Between Generic and Branded Lamotrigine in People With Epilepsy: The EQUIGEN Randomized Clinical Trial.
Berg M, Welty TE, Gidal BE, Diaz FJ, Krebill R, Szaflarski JP, Dworetzky BA, Pollard JR, Elder EJ Jr, Jiang W, Jiang X, Switzer RD, Privitera MD. Berg M, et al. JAMA Neurol. 2017 Aug 1;74(8):919-926. doi: 10.1001/jamaneurol.2017.0497. JAMA Neurol. 2017. PMID: 28654954 Free PMC article. Clinical Trial.
-
Generic-to-generic lamotrigine switches in people with epilepsy: the randomised controlled EQUIGEN trial.
Privitera MD, Welty TE, Gidal BE, Diaz FJ, Krebill R, Szaflarski JP, Dworetzky BA, Pollard JR, Elder EJ Jr, Jiang W, Jiang X, Berg M. Privitera MD, et al. Lancet Neurol. 2016 Apr;15(4):365-72. doi: 10.1016/S1474-4422(16)00014-4. Epub 2016 Feb 12. Lancet Neurol. 2016. PMID: 26875743 Clinical Trial.
-
Brand-to-generic levetiracetam switch in patients with epilepsy in a routine clinical setting.
Markoula S, Chatzistefanidis D, Gatzonis S, Siatouni A, Siarava E, Verentzioti A, Kyritsis AP, Patsalos PN. Markoula S, et al. Seizure. 2017 May;48:1-6. doi: 10.1016/j.seizure.2017.03.012. Epub 2017 Mar 19. Seizure. 2017. PMID: 28363098 Clinical Trial.
-
Generic antiepileptic drugs-Safe or harmful in patients with epilepsy?
Holtkamp M, Theodore WH.
 Holtkamp M, et al. Epilepsia. 2018 Jul;59(7):1273-1281. doi: 10.1111/epi.14439. Epub 2018 Jun 12. Epilepsia. 2018. PMID: 29894004 Review.
Holtkamp M, et al. Epilepsia. 2018 Jul;59(7):1273-1281. doi: 10.1111/epi.14439. Epub 2018 Jun 12. Epilepsia. 2018. PMID: 29894004 Review. -
Generic products of antiepileptic drugs: a perspective on bioequivalence and interchangeability.
Bialer M, Midha KK. Bialer M, et al. Epilepsia. 2010 Jun;51(6):941-50. doi: 10.1111/j.1528-1167.2010.02573.x. Epub 2010 Apr 8. Epilepsia. 2010. PMID: 20384761 Review.
See all similar articles
Cited by
-
Therapeutic Basis of Generic Substitution of Antiseizure Medications.
Elmer S, Reddy DS. Elmer S, et al. J Pharmacol Exp Ther. 2022 May;381(2):188-196. doi: 10.1124/jpet.121.000994. Epub 2022 Mar 3. J Pharmacol Exp Ther.
 2022. PMID: 35241634 Review.
2022. PMID: 35241634 Review. -
Barriers to generic antiseizure medication use: Results of a global survey by the International League Against Epilepsy Generic Substitution Task Force.
Niyongere J, Welty TE, Bell MW, Consalvo D, Hammond C, Leung H, Patsalos PN, Ryan M, Suansanae T, Zhou D, Zuellig H. Niyongere J, et al. Epilepsia Open. 2022 Jun;7(2):260-270. doi: 10.1002/epi4.12583. Epub 2022 Feb 18. Epilepsia Open. 2022. PMID: 35124903 Free PMC article.
-
Effects of generic exchange of solid oral dosage forms in neurological disorders: a systematic review.
Weitzel J, Erzkamp S, Langer K, Rose O. Weitzel J, et al. Int J Clin Pharm. 2020 Apr;42(2):393-417. doi: 10.1007/s11096-020-01023-2. Epub 2020 Apr 9.
 Int J Clin Pharm. 2020. PMID: 32274633
Int J Clin Pharm. 2020. PMID: 32274633 -
Lamotrigine add-on therapy for drug-resistant focal epilepsy.
Panebianco M, Bresnahan R, Ramaratnam S, Marson AG. Panebianco M, et al. Cochrane Database Syst Rev. 2020 Mar 20;3(3):CD001909. doi: 10.1002/14651858.CD001909.pub3. Cochrane Database Syst Rev. 2020. PMID: 32196639 Free PMC article.
-
Lack of Association of Generic Brittle Status with Genetics and Physiologic Measures in Patients with Epilepsy.
Das S, Guo D, Jiang X, Jiang W, Shu Y, Ting TY, Polli JE. Das S, et al. Pharm Res. 2020 Feb 26;37(3):60. doi: 10.1007/s11095-020-2781-6. Pharm Res. 2020. PMID: 32103380
See all "Cited by" articles
Publication types
MeSH terms
Substances
Grant support
- HHSF223201010144A/PHS HHS/United States
The funded brand of lamotrigine is changing
Published: 22 August 2019 | Updated: 15 November 2019 What's changed?
15 November 2019 widened access criteria for exceptional circumstances funding added
26 September 2019 NZTA recommendations updated
If you would like to know what changes were made when the article was updated please contact us
Key practice points:
- Three brands of lamotrigine (Lamictal, Arrow-Lamotrigine and Logem) are currently funded in 25 mg, 50 mg and 100 mg tablet strengths
- From 1 October, 2019, only one brand of lamotrigine in these tablet strengths will be funded: Logem.
 Patients prescribed
other brands will need to change to Logem; this can be done from now.
Patients prescribed
other brands will need to change to Logem; this can be done from now. - In addition, from 1 October all dispensings of lamotrigine will be three months stat (unless otherwise specified by the prescriber) so that patients can access their medicines with fewer visits to the pharmacy
- Lamotrigine is also funded in 2 mg (Lamictal) and 5 mg (Lamictal, Arrow-Lamotrigine) formulations; funding arrangements for these tablet strengths will remain the same
- Patients or caregivers require information and reassurance during a brand change as differences in the appearance of medicines may result in confusion, reduced adherence or concerns about effectiveness.
- If an additional consultation is required to assist patients with the brand change, general practices can invoice PHARMAC for the usual patient co-payment fee, so the consultation is at no cost to the patient (invoices must be received by 31 December, 2019)
- In exceptional circumstances*, clinicians can apply to PHARMAC for a patient to continue funded use of an alternative brand of lamotrigine
*Exceptional circumstances criteria widened 15/11/9
N. B. This article has been distributed to primary healthcare professionals, and specialist interest groups in New Zealand (paediatric, neurology and psychology groups)
B. This article has been distributed to primary healthcare professionals, and specialist interest groups in New Zealand (paediatric, neurology and psychology groups)
Lamotrigine is used for the treatment of epilepsy and some mood disorders
Lamotrigine is an antiepileptic medicine used in the treatment of patients with focal, generalised or absence seizures.1 In addition, it may be used in the management of some patients with bipolar disorder.2
Funding arrangements are changing
There are currently three funded brands of lamotrigine in 25 mg, 50 mg and 100 mg dispersible tablets:
- Lamictal (innovator branded medicine)
- Arrow-Lamotrigine (generic branded medicine)
- Logem (generic branded medicine)
These brands have been available fully funded without restriction for over ten years (Table 1).3 Two other
brands, Motrig and Mogine, have also been funded and delisted within this timeframe. 3
3
From 1 October, 2019, funding for two of these brands will cease and Logem will be the only funded brand of lamotrigine in these formulations.4 Therefore patients currently prescribed other brands will need to change to Logem; this can be done anytime from now, when the patient is ready to change and a new prescription is due to be dispensed (prescriptions dispensed after 1 October will be for Logem).
In addition, from 1 October, 2019 lamotrigine will be dispensed as three months’ supply stat to reduce the number of visits patients need to make to a pharmacy; if this is not an appropriate arrangement for a particular patient, prescribers can indicate a more frequent dispensing interval.4
Lamotrigine is also available in 2 mg (Lamictal) and 5 mg (Lamictal or Arrow-Lamotrigine) dispersible tablets and funding
arrangements and brands for these formulations will remain unchanged. 4
4
Dispensing data show there are approximately 12,500 patients dispensed lamotrigine tablets.5 Approximately 11,000 of these patients are dispensed Lamictal or Arrow-Lamotrigine and will need to be changed to Logem.5
Many patients have already been changing brands
Dispensing data show that approximately half of patients dispensed lamotrigine have changed brands at some point, approximately one in three have changed brands two or more times and one in ten have changed brands six or more times.5, 7
Dispensing data is unable to reveal the reasons for brand change, i.e. whether a prescriber purposely intended for a
patient to change brands, or whether patients were appropriately given a prescription using the generic medicine name,
i.e. lamotrigine, and they were dispensed an alternative brand at the pharmacy, e.g. due to attending a different pharmacy
or due to available stock. However, these data illustrate that many patients have been changing between the available
funded brands, with no specific safety issues identified. Changes in pill appearance are associated with reduced adherence.8,
9 Therefore, changing brands frequently may affect adherence and a single funded brand can reduce the potential
for this to happen.
However, these data illustrate that many patients have been changing between the available
funded brands, with no specific safety issues identified. Changes in pill appearance are associated with reduced adherence.8,
9 Therefore, changing brands frequently may affect adherence and a single funded brand can reduce the potential
for this to happen.
Table 1: Currently funded brands of lamotrigine3,6
Brand |
Date of Medsafe approval |
Funded without restriction since |
New Zealand sponsor or distributor |
|---|---|---|---|
Lamictal (innovator) |
Dec 1995 |
July 2007* |
GlaxoSmithKline (NZ) Ltd |
Arrow-Lamotrigine (generic) |
July 2006 |
Feb 2007 |
Teva Pharma (New Zealand) Ltd |
Logem (generic) |
Sept 2006 |
June 2008 |
Mylan New Zealand Ltd |
*Funded with Special Authority approval prior to this date
The evidence behind the decision to change brands
One of the key considerations for a brand change involving an antiepileptic medicine is the likelihood of this affecting
seizure control or mood stabilisation. The occurrence of seizures in epilepsy or alteration of mood control in bipolar
disorder can substantially impact the quality of life of patients and their families and caregivers. Factors which could
contribute to this, such as a change in treatment regimen, can cause understandable concern.
The occurrence of seizures in epilepsy or alteration of mood control in bipolar
disorder can substantially impact the quality of life of patients and their families and caregivers. Factors which could
contribute to this, such as a change in treatment regimen, can cause understandable concern.
Some studies have reported variations in seizure control in patients with epilepsy who change between different brands
of medicine.10, 11 However, this has not been observed in all studies and the risks of changing differ between
antiepileptic medicines.12 In the United Kingdom, the Medicines and Healthcare Products Regulatory Agency
(MHRA) proposed a classification system to help clinicians and pharmacists make appropriate decisions when considering
whether a patient could change brands (see: “The MHRA classification system”).12 Under this classification
system, there was no clear evidence at the time of assessment to place lamotrigine into either a category of medicines
for which there are clear differences in efficacy between brands or a category of medicines for which the potential for
these differences is low.
On 7 February, 2019, as part of the process for the proposed funding changes, the Neurological and Mental Health Subcommittees of the Pharmaceutical and Therapeutics Advisory Committee (PTAC) for PHARMAC held a joint meeting to discuss the evidence and consider the feedback from the sector (see: “The clinical expert advisory assessment of lamotrigine brand changing”). The clinical advisory groups recommended that changes between approved formulations of lamotrigine produced by different manufacturers would be unlikely to result in problems for patients with epilepsy or mood disorders.7 The Subcommittees recommended that a change in the funded brand of lamotrigine should proceed, with appropriate support and reassurance provided to patients during the transition.7
The clinical expert advisory assessment of lamotrigine brand changing
On 29 August, 2018, PHARMAC released a proposal to move from three funded brands of lamotrigine to one funded brand.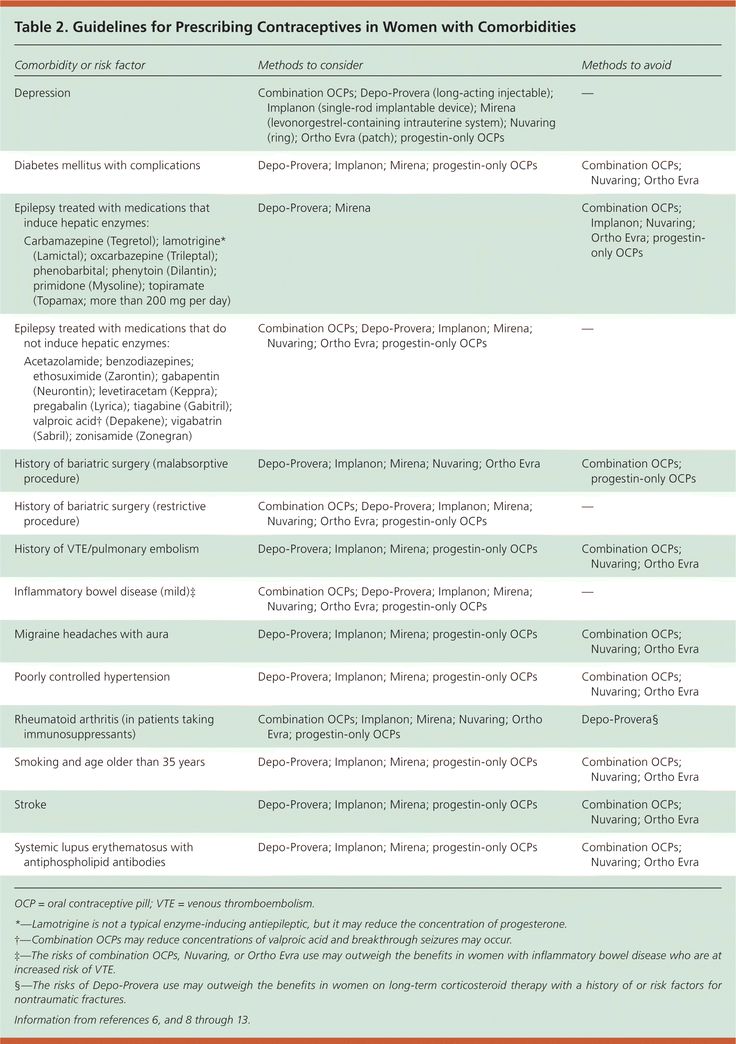 11 A
number of submissions were received providing support, feedback, concerns and suggestions, including from Medsafe, healthcare
professionals, consumers and consumer groups, and pharmaceutical suppliers.12
11 A
number of submissions were received providing support, feedback, concerns and suggestions, including from Medsafe, healthcare
professionals, consumers and consumer groups, and pharmaceutical suppliers.12
Many healthcare professional respondents were broadly supportive of the proposed changes. Concerns regarding potential differences in effectiveness, possible changes in seizure control or in the stability of mood disorders and the provision of adequate support for patients during the transition were raised in some submissions.6, 13 Some of these concerns were later emphasised in media coverage and wider publicity.14
Changing brands of lamotrigine is unlikely to affect the health of patients with epilepsy or mood disorders
Following the proposal and submissions, a clinical expert advisory group consisting of the Neurological and Mental Health
Subcommittees of the Pharmaceutical and Therapeutics Advisory Committee (PTAC) met on 7 February, 2019. At this meeting,
the Subcommittees discussed feedback regarding the proposed funding changes, reviewed publications forwarded to PHARMAC
in response to the proposal and conducted a review of evidence regarding the clinical effects of changing lamotrigine
brands, updating a previous review conducted by the Neurological Subcommittee in November, 2015.6 During
this review the Subcommittees considered over 50 publications related to the effectiveness of different brands of antiepileptic
medicines, the effects of changing brands of antiepileptic medicines (either from the innovator brand to a generic brand,
vice versa, or between different generic brands) and the bioequivalence of different brands of antiepileptic medicines.
At this meeting,
the Subcommittees discussed feedback regarding the proposed funding changes, reviewed publications forwarded to PHARMAC
in response to the proposal and conducted a review of evidence regarding the clinical effects of changing lamotrigine
brands, updating a previous review conducted by the Neurological Subcommittee in November, 2015.6 During
this review the Subcommittees considered over 50 publications related to the effectiveness of different brands of antiepileptic
medicines, the effects of changing brands of antiepileptic medicines (either from the innovator brand to a generic brand,
vice versa, or between different generic brands) and the bioequivalence of different brands of antiepileptic medicines.
On the basis of the evidence discussed at this meeting, summarised below, the PTAC Subcommittees recommended that changes
between approved formulations of lamotrigine produced by different manufacturers would be unlikely to result in problems
for the majority of patients with epilepsy or mood disorders. 6 In addition, the new funding arrangements
would stop inadvertent changing between the three funded brands that is occurring at present, which could cause confusion
or reduced adherence for patients due to unplanned alternations in the appearance of their medicine.6 The
Subcommittees recommended that a change in the funded brand of lamotrigine should proceed, with appropriate support and
reassurance provided to patients during the transition.6
6 In addition, the new funding arrangements
would stop inadvertent changing between the three funded brands that is occurring at present, which could cause confusion
or reduced adherence for patients due to unplanned alternations in the appearance of their medicine.6 The
Subcommittees recommended that a change in the funded brand of lamotrigine should proceed, with appropriate support and
reassurance provided to patients during the transition.6
Key factors considered by the Neurological and Mental Health Subcommittees of the PTAC include:6
- Three recent clinical trials, funded by the Food and Drug Administration of the United States (FDA), reported that different
brands of lamotrigine are bioequivalent in adult patients with epilepsy (see: “Bioequivalence
studies of lamotrigine”).15–17 There
are no known reasons why these medicines would not also be bioequivalent in patients aged under 18 years
with epilepsy.

- Many patients are already changing brands of lamotrigine; approximately half of all patients dispensed lamotrigine in 2018 have previously changed brands (see: “Many patients have already been changing brands”). PHARMAC have not been informed of any significant clinical impacts for these patients when they changed brands, and reports to the Centre for Adverse Reactions Monitoring (CARM) have not identified ongoing problems from brand changes.18
- Research examining health outcomes for patients in New Zealand who have changed lamotrigine brands has not identified any differences in rates of emergency department visits, hospital admissions or appointments in secondary care.19 Comparable studies conducted overseas have reported similar results.8, 20, 21
- Some patients may have a recurrence of seizures after changing brands of antiepileptic medicine, however, determining
whether this is association or causation is extremely difficult.
 Studies have reported that between 7–22% of patients
who are seizure free for two years or more experience a relapse even when continuing on the same medicine regimen.22,23
Studies have reported that between 7–22% of patients
who are seizure free for two years or more experience a relapse even when continuing on the same medicine regimen.22,23
Further details are publicly available in the Neurological and Mental Health Subcommittees’ minutes: https://pharmac.govt.nz/assets/ptac-neurological-and-mental-health-subcommittee-lamotrigine-minute-2019-02-.pdf
MHRA classification system
Classification system for antiepileptic medicines and brand changing proposed by the Commission on Human Medicines, United Kingdom, in 2013 and revised in 2017.12
Proposed category |
Description |
Funded antiepileptic medicines included in this category |
|---|---|---|
Category 1 |
Antiepileptic medicines with clear evidence of differences in efficacy between brands. |
|
Category 2 |
Antiepileptic medicines which do not clearly fit into either of the other two categories based on evidence available at the time of assessment |
|
Category 3 |
Antiepileptic medicines where the potential for clinically relevant differences between brands to exist was low |
|
For further information on the MHRA classification system, see:
www. gov.uk/drug-safety-update/antiepileptic-drugs-updated-advice-on-switching-between-different-manufacturers-products
gov.uk/drug-safety-update/antiepileptic-drugs-updated-advice-on-switching-between-different-manufacturers-products
Changing patients to another brand of lamotrigine
Discuss the brand change and address questions and concerns
Brand changes for antiepileptic medicines can be a cause of apprehension or concern for parents or caregivers. The change
in brand of lamotrigine should be discussed before issuing a prescription. A survey conducted by the Epilepsy Society
in the United Kingdom in 2014 found that three-quarters of patients experienced emotions such as confusion, anxiety, worry
or anger when changing to another brand of medicine.*26 In addition, four out of ten patients were not aware
they had been given a different brand until after they left the pharmacy. Although it is not clear how relevant these
findings are to patients in New Zealand, they highlight the need for prior discussions and clear communication before
providing patients with a different brand than they are usually prescribed or dispensed.
If the patient does not wish to change brands, they can enquire with their local pharmacy as to the cost of remaining on their preferred brand, or they may qualify for Exceptional Circumstances funding (see: In exceptional circumstances patients may continue with funded access to other brands).
* It is possible that some patients in this survey were changed to a different medicine, rather than a different brand of the same medicine
Is it safe to change brands?
Patients and caregivers can be reassured that the evidence has been thoroughly reviewed and concerns discussed extensively by the PTAC Subcommittees who concluded that there was no pharmacological reason to suggest there would be a clinical problem from changing brands of lamotrigine for the majority of patients with epilepsy or mental health conditions.7
Will the new brand be just as good?
Some patients or caregivers may worry that the formulation they are receiving will not be as safe or effective as their
current brand, e. g. it’s a “cheap alternative”.16 Patients or caregivers can be reassured that the different
brands of lamotrigine have been assessed as bioequivalent (see: “Bioequivalence studies of lamotrigine”), and Logem has
been used in New Zealand for over ten years. There may be some differences in tablet excipients between brands, but this
does not alter the clinical effect.
g. it’s a “cheap alternative”.16 Patients or caregivers can be reassured that the different
brands of lamotrigine have been assessed as bioequivalent (see: “Bioequivalence studies of lamotrigine”), and Logem has
been used in New Zealand for over ten years. There may be some differences in tablet excipients between brands, but this
does not alter the clinical effect.
Is it ok for my child to change brands?
There is no published evidence about changing brands of lamotrigine in children. Three recent clinical trials concluded
that different brands of lamotrigine are bioequivalent in adults with epilepsy (see: “Bioequivalence
studies of lamotrigine”).17–19 There
are no known reasons why these medicines would not also be bioequivalent in patients aged under 18 years with epilepsy.
Therefore, the PTAC Subcommittees did not recommend an exemption for children from the brand change. If there are concerns
around epilepsy control with brand change in a particular child, this can be discussed with a paediatrician or neurologist.
Can I combine brands?
Patients who are currently taking a regimen involving a combination of lower (e.g. 2 mg or 5 mg) and higher (e.g. 25 mg) strength lamotrigine will need to combine two different brands from 1 October, when Logem becomes the sole funded brand for higher strength tablets (lower strength formulations are Lamictal or Arrow-Lamotrigine brands). Patients or caregivers can be reassured that the different brands of lamotrigine have been assessed as bioequivalent, and there are no known issues with taking a regimen including two different brands of lamotrigine.
Will I notice any difference when I change brands?
Generic medicines are designated as bioequivalent and contain the same dose of active ingredient, however, it is possible
that some patients may absorb a slightly lower or higher amount of medicine when they change to a different brand due
to slight differences in bioavailability which are within the accepted margin of error. Although unlikely, this could
result in clinical symptoms (see: “Patient follow up”). If a patient is going to experience any symptoms as a result of
the brand change, expert opinion is that these will almost certainly occur within the first eight weeks following the change. Therefore, reassure
patients that they will receive support and follow-up after changing their brand to ensure they are not experiencing any
difficulties.
Although unlikely, this could
result in clinical symptoms (see: “Patient follow up”). If a patient is going to experience any symptoms as a result of
the brand change, expert opinion is that these will almost certainly occur within the first eight weeks following the change. Therefore, reassure
patients that they will receive support and follow-up after changing their brand to ensure they are not experiencing any
difficulties.
The nocebo effect is when patients experience symptoms that are caused by anticipating adverse effects rather than actual adverse effects of a medicine. This can be influenced by extensive media coverage of an issue or discussion on social media, as well as by the language that a health professional uses when discussing symptoms and signs to be aware of.
The nocebo effect is discussed in more detail here: www.bpac.org.nz/2019/nocebo.aspx
If I have a seizure, was it because of the brand change?
One of the key dilemmas when changing patients with epilepsy to another brand of medicine is that if seizures occur
it is difficult to determine whether a change in brand is the cause of their altered seizure control. Any seizure activity
following brand change is unlikely to be because of the change, but it cannot be ruled out. Expert opinion is that seizure activity more than
eight weeks following the change will not be as a result of the change. Some patients with epilepsy are likely to have
seizures regardless of which formulation of medicine they are prescribed and there are many other factors which may affect
seizure control, e.g. medicine adherence.
Any seizure activity
following brand change is unlikely to be because of the change, but it cannot be ruled out. Expert opinion is that seizure activity more than
eight weeks following the change will not be as a result of the change. Some patients with epilepsy are likely to have
seizures regardless of which formulation of medicine they are prescribed and there are many other factors which may affect
seizure control, e.g. medicine adherence.
Will the brand change affect my driver licence?
For people with epilepsy who drive, it is not necessary to notify the New Zealand Transport Authority (NZTA) if they change brands of lamotrigine. The NZTA have
stated that they do not regard this as a change in treatment for the purposes of a person’s fitness to drive, and consider the risk of problems from changing
brands of lamotrigine to be extremely low.7 Nevertheless, if clinicians or patients have concerns about seizure control during a brand change due to their
previous seizure history or other factors, the NZTA recommends that patients consider voluntarily avoiding driving for eight weeks. If a patient has a
seizure within the first eight weeks of a medicine brand change, application can be made to NZTA for consideration of a six-month stand down period instead of the standard 12 months.
If a patient has a
seizure within the first eight weeks of a medicine brand change, application can be made to NZTA for consideration of a six-month stand down period instead of the standard 12 months.
For further information, see: https://www.nzta.govt.nz/driver-licences/getting-a-licence/medical-requirements/information-for-health-practitioners/
Bioequivalence studies of lamotrigine
For a generic medicine to be approved for therapeutic use, manufacturers must present clinical evidence to Medsafe to demonstrate that the medicine is bioequivalent to the originator brand of medicine or a suitable alternative, known as the reference medicine.
Bioequivalence studies measure aspects of absorption and blood levels of the active ingredient including the area under
the curve (AUC), which reflects the extent and duration of exposure to the active ingredient, and the maximum plasma concentration
(Cmax). 25 For each of these, if the 90% confidence interval for the ratio of the generic and
reference medicines is within 80–125%, then the medicines are considered to be bioequivalent.25 The range
of 80–125% for medicines to be accepted as bioequivalent is the international standard, used by Medsafe and international
regulatory authorities such as the United States FDA, the European Medicines Agency and the Therapeutic Goods Administration
of Australia.26, 27 This range accounts for statistical error that is considered to be clinically insignificant.25 The
actual differences in exposure to the active ingredient between generic and reference medicines is typically less than
5%.25 Six different generic versions of lamotrigine have been approved for use in New Zealand by Medsafe
on the basis of bioequivalence studies.7
25 For each of these, if the 90% confidence interval for the ratio of the generic and
reference medicines is within 80–125%, then the medicines are considered to be bioequivalent.25 The range
of 80–125% for medicines to be accepted as bioequivalent is the international standard, used by Medsafe and international
regulatory authorities such as the United States FDA, the European Medicines Agency and the Therapeutic Goods Administration
of Australia.26, 27 This range accounts for statistical error that is considered to be clinically insignificant.25 The
actual differences in exposure to the active ingredient between generic and reference medicines is typically less than
5%.25 Six different generic versions of lamotrigine have been approved for use in New Zealand by Medsafe
on the basis of bioequivalence studies.7
Typically, bioequivalence studies are conducted in healthy patients. One concern which had been raised in relation to
antiepileptic medicines was whether results in healthy patients accurately reflect absorption in patients with epilepsy.15 To
address these concerns, three recent double-blind clinical trials were funded by the FDA in order to assess the bioequivalence
of lamotrigine in patients with epilepsy. Each study reported that generic and innovator (Lamictal) formulations of lamotrigine
were bioequivalent in patients with epilepsy.15, 16, 28 In addition, these studies included patients who
had previously experienced exacerbations of seizures after changing formulations of antiepileptic medicines, as well as
patients who were also taking medicines which induce hepatic enzymes involved in the metabolism of lamotrigine.16 No
particular concerns regarding bioequivalence in these patients were identified.
One concern which had been raised in relation to
antiepileptic medicines was whether results in healthy patients accurately reflect absorption in patients with epilepsy.15 To
address these concerns, three recent double-blind clinical trials were funded by the FDA in order to assess the bioequivalence
of lamotrigine in patients with epilepsy. Each study reported that generic and innovator (Lamictal) formulations of lamotrigine
were bioequivalent in patients with epilepsy.15, 16, 28 In addition, these studies included patients who
had previously experienced exacerbations of seizures after changing formulations of antiepileptic medicines, as well as
patients who were also taking medicines which induce hepatic enzymes involved in the metabolism of lamotrigine.16 No
particular concerns regarding bioequivalence in these patients were identified.
Further information on bioequivalence studies and the approval process for generic medicines is available at:
-
https://medsafe.
 govt.nz/profs/PUArticles/Mar2013GenericMedBioqueivalence.htm
govt.nz/profs/PUArticles/Mar2013GenericMedBioqueivalence.htm - https://www.medsafe.govt.nz/profs/PUArticles/September2017/TheMedsafeFiles4NMAssessment.htm
Advice at the pharmacy
When a brand change is implemented, pharmacists should check that patients are aware they are being dispensed a different brand than the medicine they may be used to, and address any questions or concerns. Discussion about an upcoming brand change is also useful to prepare patients for the change that will need to occur when their current brand is no longer funded. Keep in mind the sensitive nature of discussing changes to a patient’s medicines and offer them the option of talking in private.
Patients may notice a difference in the shape of their tablets
When a different brand is dispensed, pharmacists can show the patient what their new medicine looks like and emphasise
that the active ingredient remains the same. The three currently funded brands of lamotrigine have a similar appearance,
size and flavouring, with tablet shape being the main difference between brands (Table 3). Evidence suggests that changes
in the appearance of a medicine may influence adherence,8, 31 therefore this is a crucial time to ensure
that patients are aware of the brand change and to address any potential issues.
The three currently funded brands of lamotrigine have a similar appearance,
size and flavouring, with tablet shape being the main difference between brands (Table 3). Evidence suggests that changes
in the appearance of a medicine may influence adherence,8, 31 therefore this is a crucial time to ensure
that patients are aware of the brand change and to address any potential issues.
Table 3: Currently funded formulations of 25 mg, 50 mg, and 100mg lamotrigine tablets and their appearance
Logem32 |
Lamictal33 |
Arrow-Lamotrigine34 |
|
|---|---|---|---|
Pack size |
56 tablet blister pack |
56 tablet blister pack |
56 tablet blister pack |
Flavour |
Blackcurrant |
Blackcurrant |
Blackcurrant |
Sweetener |
Saccharin and mannitol |
Saccharin |
Saccharin and mannitol |
Writing on tablets |
“LY” with dose, e. |
25 mg: “GSCL5” on one side, “25” on the other 50 mg: “GSCX7” on one side, “50” on the other 100 mg: “GSCL7” on one side, “100” on the other |
Embossed with an outline similar to a PacMan with “LI” and the dose underneath, e.g “LI25” for a 25 mg tablet. The reverse side has a line marked on it. |
Tablet appearance |
White to off-white Round with a flat face |
White to off-white, rounded edges with a raised centre |
White to off-white Shield-shaped |
25 mg |
|||
50 mg |
|||
100 mg |
|||
Tablet size |
Tablet size increases with strength. |
||
Patient follow-up
Patients should be followed up after changing from Lamictal or Arrow-Lamotrigine branded tablets to Logem to check on medicine adherence and whether patients or caregivers have any concerns regarding the alteration in medicine regimen.
If clinical symptoms and signs have emerged since the brand change, consider whether they could be caused by small differences in absorption of medicine with the new brand, or whether other factors could explain the occurrence, e.g. usual variation in the patient’s clinical condition, changes in the patient’s environment or daily activities, anxiety about the brand change and anticipation of symptoms.
Symptoms which could indicate that patients are absorbing an increased dose of lamotrigine include:
7- Headache
- Nausea
- Tremor
- Dizziness
- Irritability
- Blurred vision or visual disturbances
If these symptoms occur, consider requesting a serum lamotrigine level and discuss with a neurologist, paediatrician
or psychiatrist as appropriate.
Symptoms which could indicate that patients are absorbing a reduced dose of lamotrigine or have reduced adherence include:
7- Seizures/aura – N.B. it is now considered that aura is part of a seizure event and should be treated as such
- Myoclonic jerks
- Mood instability (in patients taking lamotrigine for mood disorders)
If symptoms occur, consider increasing the dose of lamotrigine by 25 mg in an adult or child aged over 12 years or by 300 micrograms/kg in a child aged 2 – 12 years, as long as the total dose remains below the recommended maximum dose, which differs depending on other anti-epileptic medicines taken and the indication for treatment: see NZF for recommended maximum doses in adults: www.nzf.org.nz/nzf_2326 or NZFC for recommended doses in children: www.nzfchildren.org.nz/nzf_2326
If the patient is already taking the maximum dose of lamotrigine or the dose increase does not improve symptoms, discuss
with or refer to a neurologist, paediatrician or psychiatrist as appropriate.
Routine monitoring of serum levels is not necessary
The Neurological and Mental Health Subcommittees of PTAC considered whether monitoring serum levels of lamotrigine would
aid in clinical management during a brand change, however, they concluded that based on available evidence this would
be of little benefit as monitoring is generally used to check for adherence, possible toxicity or during pregnancy. Clinical
trials have found that there is some dose to dose variation in serum levels of lamotrigine; importantly, these variations
are similar for patients who change from the originator brand to a generic formulation of lamotrigine and patients who
continue to take the originator brand.17, 36 In addition, as most patients would be expected to continue
with good adherence to their regimen it is unlikely that monitoring would aid clinical decision making when patients are
changed between brands of lamotrigine. 7
7
Some patients with epilepsy are likely to have seizures regardless of which formulation they are prescribed
If a patient with epilepsy experiences a seizure after changing brands of lamotrigine, it is possible that this is related to the brand change, but also possible that this was a random occurrence or influenced by other factors, such as acute illnesses, co-morbidities, changes in daily routine or other medicines which may affect seizure control or the efficacy of antiepileptic medicines. Evidence from two randomised clinical trials report that between 7–22% of patients can have a recurrence while continuing the same antiepileptic medicine regimen even after two years without seizures.24, 25
Discuss with patients that a change in seizure control may not be related to the brand change, and it does not necessarily
mean that the Logem brand should be discontinued. If appropriate, consult with a neurologist involved in managing the
patient’s epilepsy.
If needed, funding to cover the cost of a follow-up appointment is available
If patients are having difficulty after being changed to Logem and require an additional appointment to discuss concerns, clinicians can invoice PHARMAC for the General Practitioner co-payment fee (i.e. in lieu of charging the patient) as long as the invoice is received on or prior to 31 December, 2019.4, 37
For further information on claiming reimbursement, see:
- “Information for healthcare professionals” at https://pharmac.govt.nz/medicine-funding-and-supply/medicine-notices/lamotrigine/
In exceptional circumstances patients may continue with funded access to other brands
Funding is available to assist patients in accessing a non-funded brand of lamotrigine if there are exceptional clinical
difficulties. This could include a patient who clinicians believe would be unable to manage a change in brand, e. g. they
have had adverse clinical effects from previous brand changes, or where a patient has changed brands to Logem and has
not been able to tolerate the change.4
g. they
have had adverse clinical effects from previous brand changes, or where a patient has changed brands to Logem and has
not been able to tolerate the change.4
On 15th November, 2019, the application criteria were widened to allow prescribers to make an application to PHARMAC for ongoing funding for individuals:
- Who have not tolerated the change
- Who have breakthrough seizures
- Who have had mood destabilisation
- The prescriber has clinical concerns about the individuals ability to manage the change (e.g. previous issues with medication changes, severe anxiety around this brand change)
- That have concerns about their ability to drive
An application form for clinicians to apply for exceptional circumstances is available at: https://pharmac.govt.nz/assets/lamotrigine-exceptional-circumstances-form.doc
If patients wish to continue taking another brand of lamotrigine at their own personal cost after 1 October, 2019, they
should discuss the price and availability of supplies with a pharmacist. 4
4
Lamotrigine
Lamotrigine is an anticonvulsant drug used to treat various forms of epilepsy and mood disorders in bipolar disorder.
Synonyms Russian
Lamictal, lameptil, convulsan, lamitor, lamolep, lamotriks, seizar, triginet.
English synonyms
Lamotrigin, Lamictal.
Test Method
Gas Chromatography-Mass Spectrometry (GC-MS). nine0003
Units
μg/mL (micrograms per milliliter).
What biomaterial can be used for research?
Venous blood.
How to properly prepare for an examination?
- Do not eat for 2-3 hours before the examination, you can drink pure non-carbonated water.
- Children under 1 year of age should not eat for 30-40 minutes prior to testing. nine0039 Do not smoke for 30 minutes before the test.
Overview of the study
Lamotrigine is a broad-spectrum antiepileptic drug for various forms of epilepsy that may have beneficial effects on mood in bipolar disorders. The drug is used as mono- or additional therapy for partial and generalized convulsive seizures, seizures with Lennox-Gastaut syndrome, with typical absences, as well as for the prevention of mood disorders (depression, mania, hypomania) in patients with bipolar disorder. In addition, lamotrigine is being considered for migraine, trigeminal neuralgia, and treatment-refractory depression. nine0003
The drug is used as mono- or additional therapy for partial and generalized convulsive seizures, seizures with Lennox-Gastaut syndrome, with typical absences, as well as for the prevention of mood disorders (depression, mania, hypomania) in patients with bipolar disorder. In addition, lamotrigine is being considered for migraine, trigeminal neuralgia, and treatment-refractory depression. nine0003
Its pharmacological action is associated with the blocking of voltage-gated sodium channels, stabilization of the nerve cell membrane and suppression of the release of glutamic acid.
The bioavailability of lamotrigine is very high at 98%. After oral administration, the drug is quickly and fairly completely absorbed from the gastrointestinal tract, food intake slows down the absorption process, but does not reduce its effectiveness. The maximum concentration in the blood is observed 2.5 hours after ingestion. Binding to blood proteins is not more than 55%. Therapeutic concentration in the blood is 2. 5-15 mcg / ml. Lamotrigine is metabolized in the liver by conjugation with glucuronic acid using the enzyme glucuronyl transferase and without the participation of the cytochrome P450 system. The elimination half-life of lamotrigine is 24-35 hours and depends on the co-administered medicinal products. The drug is excreted from the body mainly by the kidneys in the form of glucuronides or unchanged (10%), and about 2% - through the intestines. nine0003
5-15 mcg / ml. Lamotrigine is metabolized in the liver by conjugation with glucuronic acid using the enzyme glucuronyl transferase and without the participation of the cytochrome P450 system. The elimination half-life of lamotrigine is 24-35 hours and depends on the co-administered medicinal products. The drug is excreted from the body mainly by the kidneys in the form of glucuronides or unchanged (10%), and about 2% - through the intestines. nine0003
While taking lamotrigine, headache, fatigue, drowsiness or insomnia, nausea, vomiting, a decrease in the number of leukocytes and platelets in the blood, skin rashes and allergic reactions may occur, in rare cases, Stevens-Johnson syndrome, Lyell's syndrome may develop. In some cases, abrupt discontinuation of the drug can provoke epileptic seizures, so when you cancel lamotrigine, the dose should be reduced gradually.
The concentration of the drug in the blood should be monitored and corrected in case of hepatic and renal insufficiency, while taking with other drugs. It is also important to consider fluctuations in blood levels of lamotrigine during pregnancy and postpartum ( Fetal Category FDA - C). During pregnancy, there is a decrease in the concentration of lamotrigine in the blood due to an increase in body weight and increased excretion under the influence of estrogens. This circumstance can lead to a worsening of the course of the disease. On the other hand, after childbirth, due to the reverse development of the above processes, physiological conditions are created for the appearance of signs of intoxication. Therefore, when planning pregnancy in women taking lamotrigine, it is advisable to determine the level of the drug in the blood and monitor it during pregnancy and after childbirth. nine0003
It is also important to consider fluctuations in blood levels of lamotrigine during pregnancy and postpartum ( Fetal Category FDA - C). During pregnancy, there is a decrease in the concentration of lamotrigine in the blood due to an increase in body weight and increased excretion under the influence of estrogens. This circumstance can lead to a worsening of the course of the disease. On the other hand, after childbirth, due to the reverse development of the above processes, physiological conditions are created for the appearance of signs of intoxication. Therefore, when planning pregnancy in women taking lamotrigine, it is advisable to determine the level of the drug in the blood and monitor it during pregnancy and after childbirth. nine0003
What is research used for?
- Blood drug concentration monitoring;
- drug interaction assessment;
- diagnosis of overdose;
- detection of violations of the drug regimen.

When is the examination scheduled?
- If symptoms persist or worsen while taking lamotrigine;
- while prescribing other anticonvulsants that affect the concentration of lamotrigine in the blood; nine0040
- if the patient has impaired liver and kidney function;
- before and during pregnancy and after childbirth;
- for adverse events likely to be related to lamotrigine and suspected drug overdose.
What do the results mean?
Reference values: 4 - 10 µg/ml.
What can influence the result?
- The concentration of lamotrigine in the blood increases: nine0038
- when taking valproic acid;
- for violations of the liver and kidneys.

Important Notes
- It is recommended to take blood for research 30-60 minutes before the scheduled intake of the drug.
- Treatment, dose adjustment of this drug and concentration monitoring is carried out strictly under the supervision of a physician. It is unacceptable to independently change the mode of administration and dose of the drug.
is also recommended
[02-014] General blood test
[06-003] Alaninaminotransferase (ALT)
[06-010] Aspartetaminotransferase (AST)
[06-036] Bilirubin total
[ 06-021] Serum creatinine
[15-001] Valproic acid
[15-006] Topiramate
Who orders the test?
Psychiatrist, psychotherapist, neurologist, clinical pharmacologist.
Literature
- Adab N. Therapeutic monitoring of antiepileptic drugs during pregnancy and in the postpartum period: is it useful? CNS Drugs.
 2006;20(10):791–800.
2006;20(10):791–800. - Cassels C. Lowering of Lamotrigine blood levels linked to increased seizure risk during pregnancy. Medscape. Nov 28, 2007.
- Johannessen SI, Landmark CJ: Value of therapeutic drug monitoring in epilepsy. Expert Rev Neurother 2008;8(6):929-939.
- Noskova T. Yu. Lamotrigine in the treatment of adult epilepsy. // Russian medical journal. - 2007. - T. 15, No. 24. - S. 1838-1841.
Analysis for drug monitoring Lamotrigine (Lamiktal) in Sochi (Adler) and medical laboratory OPTIMUM
Get test results
- Home
- Analyzes and prices
- Lamictal (Lamotrigine)
More about the doctor
Deadline
(working days):
up to 10 days.
Price:
1650 ₽ *
* Taking biomaterial is paid separately
nine0184 Pharmaceutical drug Lamictal (Lamotrigine) is prescribed for treatment of epilepsy and bipolar affective disorders, and is also included in the regimen for the treatment of depression as an adjuvant.
Lamictal has relatively few adverse effects and is well tolerated by most patients. When taken orally, the active substance is completely absorbed from the digestive tract. The highest plasma level is observed after 2.5 hours. nine0003
Up to 55% interacts with plasma proteins. Once in the body, Lamotrigine is metabolized. Metabolites are excreted mostly in the urine, only 8% are excreted unchanged. The average elimination half-life in adults is 29 hours, in children it is shorter. The pharmacokinetics of the drug varies widely, depending on the work of the patient's kidneys, while taking other medications.
When carbamazepine or phenytoin are included in the regimen along with lamotrigine, there is a decrease in half-life of lamotrigine . Sodium valproate inhibits microsomal liver enzymes, therefore, when combined with lamotrigine, a slowdown in the metabolism of the latter and an increase in its half-life (half-life) are observed.
Undesirable effects may occur if the concentration of the drug is increased above 15 mg/ml.
Undesirable effects when taking Lamotrigine
-
From the side of the nervous system: headaches, dizziness, lethargy, sleep problems, weakness, aggressiveness, confusion, tremor, involuntary eye movements, hallucinations. nine0003
-
From the gastrointestinal tract: nausea, loose stools, disorders of the liver.
-
On the part of hematopoiesis: a decrease in leukocytes and platelets.
-
Allergic manifestations: skin rashes, angioedema, malignant exudative erythema, Lyell's syndrome, lymphadenopathy.
-
From the locomotive system: pain in the back and joints, lupus-like syndrome.
Preparation for drug monitoring Lamictal
-
The analysis is prescribed either 2.5 hours before taking the pharmaceutical preparation or 2.5 hours after taking it.
-
You must bring a sample of the medication you are taking for testing.

Indications for the appointment of the study
- When the selection of a personal dosage of at the beginning of treatment.
- Concomitant administration of other medicines for detection of drug interactions .
- If a patient has physiological or pathological factors that change the pharmacokinetics of the drug, for example, the pathology of the genitourinary system, the period of pregnancy.
- If no therapeutic effect .
- When changing the dosage of a pharmaceutical product.
- When replacing the drug with its structural analogue.
Interpretation of Lamictal (Lamotrigine) monitoring results
The safety corridor is 4 to 10 mg/mL. Increased results can be observed due to a high dose of the drug or due to the peculiarities of pharmacokinetics, for example, due to the simultaneous administration of other medications or the individual characteristics of the patient's body.

 g. “25”, “50” or “100”
g. “25”, “50” or “100” The tablet sizes for each strength are similar between brands.35
The tablet sizes for each strength are similar between brands.35