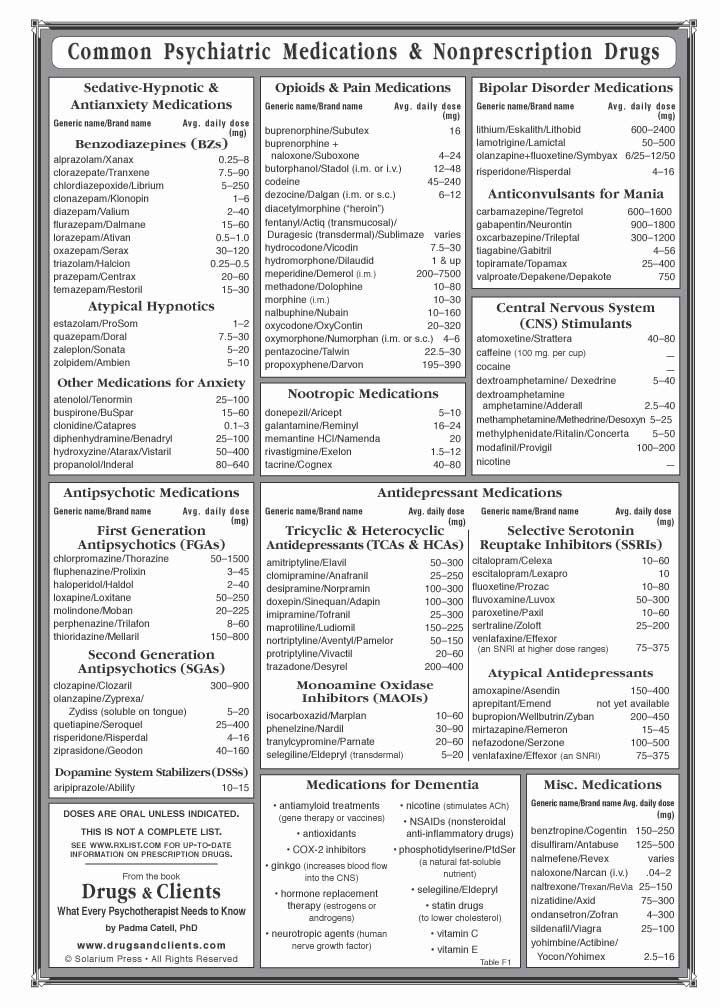
When should you take abilify
Aripiprazole (Abilify) | NAMI: National Alliance on Mental Illness
Brand names:
-
- Tablet: 2 mg, 5mg, 10 mg, 15 mg, 20 mg, 30 mg
- Abilify MyCite®
- Oral tablet with a digestible sensor: 2 mg, 5 mg, 10 mg, 15 mg, 20 mg, 30 mg
- Abilify Maintena®
- Extended-release injectable suspension: 300 mg, 400 mg
- Aristada® (aripiprazole lauroxil)
- Extended-release injectable suspension: 441 mg, 662 mg, 882 mg, 1064 mg
- Aristada Initio® (aripiprazole lauroxil)
- Extended-release injectable suspension: 675 mg
Generic name: aripiprazole (ay ri PIP ray zole), aripiprazole lauroxil (law rox il)
- Aripiprazole
- Oral tablet: 2 mg, 5mg, 10 mg, 15 mg, 20 mg, 30 mg
- Orally disintegrating tablet: 10 mg, 15 mg
- Oral solution: 1 mg/mL
All FDA black box warnings are at the end of this fact sheet. Please review before taking this medication.
What Is Aripiprazole And What Does It Treat?
Aripiprazole is a medication that works in the brain to treat schizophrenia. It is also known as a second-generation antipsychotic (SGA) or atypical antipsychotic. Aripiprazole rebalances dopamine and serotonin to improve thinking, mood, and behavior.
Symptoms of schizophrenia include:
- Hallucinations – imagined voices or images that seem real
- Delusions - beliefs that are not true (e.g., other people are reading your thoughts)
- Disorganized thinking or trouble organizing your thoughts and making sense
- Little desire to be around other people
- Trouble speaking clearly
- Lack of motivation
Aripiprazole may help some or all of these symptoms.
Aripiprazole is also FDA approved for the following indications:
- Acute treatment of manic or mixed episodes of bipolar disorder (when used alone or with lithium or valproate)
- Maintenance (long-term) treatment of bipolar disorder
- Adjunctive treatment of major depressive disorder.
 This means aripiprazole is used in addition to an antidepressant to help treat depression.
This means aripiprazole is used in addition to an antidepressant to help treat depression. - Irritability associated with autistic disorders
- Tourette’s syndrome
This medication sheet will focus primarily on schizophrenia. You can find more information about bipolar disorder, depression, and autism spectrum disorders here.
Aripiprazole may also be helpful when prescribed “off-label” for borderline personality disorder or drug-induced hyperprolactinemia (elevated prolactin levels caused by other antipsychotics). “Off-label” means that it hasn’t been approved by the Food and Drug Administration for this condition. Your mental health provider should justify his or her thinking in recommending an “off-label” treatment. They should be clear about the limits of the research around that medication and if there are any other options.
What Is The Most Important Information I Should Know About Aripiprazole?
Schizophrenia requires long-term treatment. Do not stop taking aripiprazole, even when you feel better.
Do not stop taking aripiprazole, even when you feel better.
With input from you, your health care provider will assess how long you will need to take the medicine.
Missing doses of aripiprazole may increase your risk for a relapse in your symptoms.
Do not stop taking aripiprazole or change your dose without talking with your health care provider first.
For aripiprazole to work properly, it should be taken every day as ordered by your health care provider.
Are There Specific Concerns About Aripiprazole And Pregnancy?
If you are planning on becoming pregnant, notify your health care provider to best manage your medications. People living with schizophrenia who wish to become pregnant face important decisions. This is a complex decision since untreated schizophrenia has risks to the fetus, as well as the mother. It is important to discuss the risks and benefits of treatment with your doctor and caregivers.
Antipsychotic use during the third trimester of pregnancy has a risk for abnormal muscle movements (extrapyramidal symptoms [EPS]) and/or withdrawal symptoms in newborns following delivery. Symptoms in the newborn may include agitation, feeding disorder, hypertonia, hypotonia, respiratory distress, somnolence, and tremor; these effects may be self-limiting or require hospitalization.
Symptoms in the newborn may include agitation, feeding disorder, hypertonia, hypotonia, respiratory distress, somnolence, and tremor; these effects may be self-limiting or require hospitalization.
In general, infants exposed to SGAs via breast milk should be monitored weekly for the first month of exposure for symptoms, such as appetite changes, insomnia, irritability, or lethargy.
Caution is advised with breastfeeding since aripiprazole does pass into breast milk.
What Should I Discuss With My Health Care Provider Before Taking Aripiprazole?
- Symptoms of your condition that bother you the most
- If you have thoughts of suicide or harming yourself
- Medications you have taken in the past for your condition, whether they were effective or caused any adverse effects
- If you ever had muscle stiffness, shaking, tardive dyskinesia, neuroleptic malignant syndrome, or weight gain caused by a medication
- If you experience side effects from your medications, discuss them with your provider.
 Some side effects may pass with time, but others may require changes in the medication.
Some side effects may pass with time, but others may require changes in the medication. - Any psychiatric or medical problems you have, such as heart rhythm problems, long QT syndrome, heart attacks, diabetes, high cholesterol, or seizures
- If you have a family history of diabetes or heart disease
- All other medications you are currently taking (including over the counter products, herbal and nutritional supplements) and any medication allergies you have
- Other non-medication treatment you are receiving, such as talk therapy or substance abuse treatment. Your provider can explain how these different treatments work with the medication.
- If you are pregnant, plan to become pregnant, or are breastfeeding
- If you smoke, drink alcohol, or use illegal drugs
How Should I Take Aripiprazole?
Aripiprazole tablets and suspension are usually taken 1 time per day with or without food.
Typically, patients begin at a low dose of medicine and the dose is increased slowly over several weeks.
The oral dose of aripiprazole usually ranges from 2 mg to 30 mg taken once daily. The dose of aripiprazole maintena extended-release injection ranges from 300 mg to 400 mg given once monthly; the dose of aripiprazole lauroxil extended-release injection ranges from 441 mg to 1064 mg – depending on the dose, it is given once per month, every 6 weeks, or every 2 months. Aripiprazole Maintena requires a two-week oral medication overlap; aripiprazole lauroxil requires a three-week oral overlap. Aripiprazole lauroxil Initio is typically given after an oral dose of aripiprazole along with an aripiprazole lauroxil injection – this allows for a one-day initiation and does not require further oral overlap. Only your health care provider can determine the correct formulation and dose for you.
Use a calendar, pillbox, alarm clock, or cell phone alert to help you remember to take your medication. You may also ask a family member or a friend to remind you or check in with you to be sure you are taking your medication.
What Happens If I Miss A Dose Of Aripiprazole?
If you miss a dose of oral aripiprazole, take it as soon as you remember, unless it is closer to the time of your next dose. Do not double your next dose or take more than what is prescribed. If you miss an injection, call your doctor or pharmacist right away. Discuss this with your health care provider.
What Should I Avoid While Taking Aripiprazole?
Avoid drinking alcohol or using illegal drugs while you are taking aripiprazole. They may decrease the benefits (e.g., worsen your confusion) and increase adverse effects (e.g., sedation) of the medication.
What Happens If I Overdose With Aripiprazole?
If an overdose occurs call your doctor or 911. You may need urgent medical care. You may also contact the poison control center at 1-800-222-1222.
A specific treatment to reverse the effects of aripiprazole does not exist.
What Are Possible Side Effects Of Aripiprazole?
Common side effects
Headache, extrapyramidal symptoms, drowsiness, restlessness, fatigue, sedation, agitation, insomnia, anxiety, weight gain, cholesterol abnormalities, increased glucose, nausea, vomiting, constipation, application site rash (MyCite), tremor
Rare/serious side effects
Rash, dry mouth, muscle aches, seizure, agitation
Aripiprazole may increase the blood levels of a hormone called prolactin. Side effects of increased prolactin levels include females losing their period, production of breast milk and males losing their sex drive or possibly experiencing erectile problems. Long term (months or years) of elevated prolactin can lead to osteoporosis or increased risk of bone fractures.
Side effects of increased prolactin levels include females losing their period, production of breast milk and males losing their sex drive or possibly experiencing erectile problems. Long term (months or years) of elevated prolactin can lead to osteoporosis or increased risk of bone fractures.
Some people may develop muscle-related side effects while taking aripiprazole. The technical terms for these are “extrapyramidal symptoms” (EPS) and “tardive dyskinesia” (TD). Symptoms of EPS include restlessness, tremor, and stiffness. TD symptoms include slow or jerky movements that one cannot control, often starting in the mouth with tongue rolling or chewing movements.
Temperature regulation: Impaired core body temperature regulation may occur; caution with strenuous exercise, heat exposure, and dehydration.
Second generation antipsychotics (SGAs) increase the risk of weight gain, high blood sugar, and high cholesterol. This is also known as metabolic syndrome. Your health care provider may ask you for a blood sample to check your cholesterol, blood sugar, and hemoglobin A1c (a measure of blood sugar over time) while you take this medication.
Your health care provider may ask you for a blood sample to check your cholesterol, blood sugar, and hemoglobin A1c (a measure of blood sugar over time) while you take this medication.
- Information on healthy eating and adding exercise to decrease your chances of developing metabolic syndrome may be found at the following sites:
- http://www.helpguide.org/articles/healthy-eating/healthy-eating.htm
- http://www.helpguide.org/home-pages/exercise-fitness.htm
SGAs have been linked with higher risk of death, strokes, and transient ischemic attacks (TIAs) in elderly people with behavior problems due to dementia.
All antipsychotics have been associated with the risk of sudden cardiac death due to an arrhythmia (irregular heart beat). To minimize this risk, antipsychotic medications should be used in the smallest effective dose when the benefits outweigh the risks. Your doctor may order an EKG to monitor for irregular heartbeat.
Neuroleptic malignant syndrome is a rare, life threatening adverse effect of antipsychotics which occurs in <1% of patients. Symptoms include confusion, fever, extreme muscle stiffness, and sweating. If any of these symptoms occur, contact your health care provider immediately.
Symptoms include confusion, fever, extreme muscle stiffness, and sweating. If any of these symptoms occur, contact your health care provider immediately.
All antipsychotics can cause sedation, dizziness, or orthostatic hypotension (a drop in blood pressure when standing up from sitting or lying down). These side effects may lead to falls which could cause bone fractures or other injuries. This risk is higher for people with conditions or other medications that could worsen these effects. If falls or any of these symptoms occur, contact your health care provider.
The U.S. Food and Drug Administration (FDA) is warning that compulsive or uncontrollable urges to gamble, binge eat, shop, and have sex have been reported with the use of aripiprazole (Abilify, Abilify Maintena, Aristada, Aristada Initio). These uncontrollable urges were reported to have stopped when the medicine was discontinued or the dose was reduced. These impulse-control problems are rare, but they may result in harm to the patient and others if not recognized.
Are There Any Risks For Taking Aripiprazole For Long Periods Of Time?
Tardive dyskinesia (TD) is a side effect that develops with prolonged use of antipsychotics. Medications such as aripiprazole have been shown to have a lower risk of TD compared to older antipsychotics, such as Haldol® (haloperidol). If you develop symptoms of TD, such as grimacing, sucking, and smacking of lips, or other movements that you cannot control, contact your health care provider immediately. All patients taking either first or second generation antipsychotics should have an Abnormal Involuntary Movement Scale (AIMS) completed regularly by their health care provider to monitor for TD.
Second generation antipsychotics (SGAs) increase the risk of diabetes, weight gain, high cholesterol, and high triglycerides. (See “Serious Side Effects” section for monitoring recommendations).
What Other Medications May Interact With Aripiprazole?
The following medications may increase the levels and effects of aripiprazole:
- The antibiotic clarithromycin (Biaxin®)
- Antidepressants, such as fluoxetine (Prozac®), paroxetine (Paxil®), and nefazodone
- Antifungals, such as fluconazole (Diflucan®), ketoconazole (Nizoral®), and itraconazole (Sporanox®)
- The antiarrhythmic agent quinidine
- HIV medications, such as the protease inhibitors indinavir (Crixivan®), ritonavir (Norvir®), saquinavir (Fortovase®, Invirase®), and lopinavir/ritonavir (Kaletra®)
The following medications may decrease the levels and effects of aripiprazole: carbamazepine (Tegretol®) and rifampin (Rifadin®).
How Long Does It Take For Aripiprazole To Work?
It is very important to tell your doctor how you feel things are going during the first few weeks after you start taking aripiprazole. It will probably take several weeks to see big enough changes in your symptoms to decide if aripiprazole is the right medication for you.
Antipsychotic treatment is generally needed lifelong for persons with schizophrenia. Your doctor can best discuss the duration of treatment you need based on your symptoms and illness.
- Hallucinations, disorganized thinking, and delusions may improve in the first 1-2 weeks
- Sometimes these symptoms do not completely go away
- Motivation and desire to be around other people can take at least 1-2 weeks to improve
- Symptoms continue to get better the longer you take aripiprazole
- It may take 2-3 months before you get the full benefit of aripiprazole
Summary of FDA Black Box Warnings
Increased mortality in elderly patients with dementia related psychosis
- Both first generation (typical) and second generation (atypical) antipsychotics are associated with an increased risk of mortality in elderly patients when used for dementia related psychosis.

- Although there were multiple causes of death in studies, most deaths appeared to be due to cardiovascular causes (e.g. sudden cardiac death) or infection (e.g., pneumonia).
- Antipsychotics are not indicated for the treatment of dementia-related psychosis.
Suicidal Thoughts or Actions in Children, Teens, and Young Adults
Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide.
- Patients treated with antidepressants may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking medications. This risk may persist until significant remission occurs.
- Patients, their families, and caregivers should be alert to the emergence of anxiety, restlessness, irritability, aggressiveness and insomnia. If these symptoms emerge, they should be reported to the patient’s prescriber or health care professional.
- All patients being treated with this medication for depression should watch for and notify their health care provider for worsening symptoms, suicidality and unusual changes in behavior, especially during the first few months of treatment.
Provided by
(November 2022)
©2022 The College of Psychiatric and Neurologic Pharmacists (CPNP) and the National Alliance on Mental Illness (NAMI). CPNP and NAMI make this document available under the Creative Commons Attribution-No Derivatives 4.0 International License. Last Updated: January 2016.
This information is being provided as a community outreach effort of the College of Psychiatric and Neurologic Pharmacists. This information is for educational and informational purposes only and is not medical advice. This information contains a summary of important points and is not an exhaustive review of information about the medication. Always seek the advice of a physician or other qualified medical professional with any questions you may have regarding medications or medical conditions. Never delay seeking professional medical advice or disregard medical professional advice as a result of any information provided herein. The College of Psychiatric and Neurologic Pharmacists disclaims any and all liability alleged as a result of the information provided herein.
Always seek the advice of a physician or other qualified medical professional with any questions you may have regarding medications or medical conditions. Never delay seeking professional medical advice or disregard medical professional advice as a result of any information provided herein. The College of Psychiatric and Neurologic Pharmacists disclaims any and all liability alleged as a result of the information provided herein.
Taking ABILIFY® (aripiprazole)
As with any medicine, it's important to take ABILIFY (aripiprazole) exactly as directed, for as long as your doctor prescribes it. Here is some information that can help.
Talk to your doctor about all your medicines
Be sure to tell your doctor about any other medications you're on including prescription medicines, non-prescription medicines, herbal supplements, and vitamins. ABILIFY may interact with other medications, which could affect the way that each one works. Your doctor can tell you if it is safe to take ABILIFY with your other medicines. He or she can also tell you if you should start or stop any other medications while you’re on ABILIFY.
He or she can also tell you if you should start or stop any other medications while you’re on ABILIFY.
If you are using a
Savings Card to pay as little as $5 per month,* tell your pharmacist you want brand-name ABILIFY for every refillRemember: your Savings Card only works for brand-name ABILIFY. Present your Savings Card (along with your prescription for brand-name ABILIFY) to your pharmacy. If your prescription was sent electronically to your pharmacy, check to make sure your ABILIFY prescription was not switched to a generic option. If it was switched, ask the pharmacist to reprocess your prescription for brand-name ABILIFY. Then, each time you pick up, check your prescription bottle and receipt to ensure you received brand-name ABILIFY and paid $5.* Pharmacy rules or local laws may apply.
*Terms and conditions apply. Assumes one 30-day supply prescription per month. If more than one prescription is filled in a calendar month, you may pay more than $5 in that month. See other terms and conditions.
See other terms and conditions.
Report any side effects to your doctor
Everyone responds differently to medication. Serious side effects have been reported with ABILIFY. Take note of any side effects you experience, and report them to your doctor. This will help your doctor understand how to manage your side effects.
Call your doctor if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- Thoughts about suicide or dying
- Attempts to commit suicide
- New or worse depression
- New or worse anxiety
- Feeling very agitated or restless
- Panic attacks
- Trouble sleeping (insomnia)
- New or worse irritability
- Acting aggressive, being angry, or violent
- Acting on dangerous impulses
- An extreme increase in activity and talking (mania)
- Other unusual changes in behavior or mood
Know what to avoid while taking ABILIFY
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how ABILIFY affects you.
 ABILIFY may make you drowsy.
ABILIFY may make you drowsy. - Avoid getting over-heated or dehydrated
- Do not over-exercise
- In hot weather, stay inside in a cool place if possible.
- Stay out of the sun. Do not wear too much or heavy clothing
- Drink plenty of water
Stay on schedule
ABILIFY is usually taken once a day, with or without food. You may want to ask your doctor for advice on what time of day to take ABILIFY. But remember to always take ABILIFY as directed by your doctor.
Taking ABILIFY regularly may help you get the best results from your treatment. And to help yourself stay on your prescribed schedule, there are a few things you may find helpful:
- Try making your medication part of your daily routine
- Mark your dose on a calendar to-do list
- Use a childproof weekly pillbox to keep your ABILIFY pills in order
- Ask a friend or family member to help remind you
Don't stop taking ABILIFY without talking to your doctor
Even if you start to feel better, it's important to keep taking ABILIFY as prescribed by your doctor. It may take time to feel the effect of ABILIFY, so don't change your routine or stop taking ABILIFY without talking to your doctor first.
It may take time to feel the effect of ABILIFY, so don't change your routine or stop taking ABILIFY without talking to your doctor first.
Know what to do if you miss a dose
If you miss a dose, take your ABILIFY as soon as you remember. However, if it's almost time for your next dose, skip the missed dose and continue your regular schedule. Do not take a double dose. If you miss several doses, talk to your doctor right away.
Keep your doctor informed
Consider using a journal to record how you're feeling each day so you can compare it to how you felt before taking ABILIFY. Be sure to share your results with your doctor, including any side effects. This way, your doctor will better understand what effect your treatment is having and be able to adjust it if necessary.
Tell your doctor if you are pregnant or plan to become pregnant. It is not known if ABILIFY will harm your unborn baby. If you are breast feeding or plan to breast feed, talk to your doctor.
Make the most of your support system
Family and friends can provide a helping hand and be a much-needed source of encouragement. Don't be afraid to reach out, and accept support when you need it.
Don't be afraid to reach out, and accept support when you need it.
Questions? Find answers in this FAQ or call 1-888-922-4543
Abilify | Health News | Search and order medicines in pharmacies of St. Petersburg and the Leningrad region.
acmespb 11/17/2016 0 Comments
Find the drug in pharmacies
The trade name of the drug: Abilifai
International unprofatored name: aripiprazole
Dosage form: tablets at 5 mg
,0002 Active substance: Aripiprazole
Pharmacotherapeutic group: Antipsychotic
Pharmacological properties: Antipsychotic Abilify is rapidly absorbed, after ingestion, the ability of the drug to be absorbed in the human body is very high. It is 87%. After 3-5 hours, the maximum concentration of the drug is observed in plasma, but the equilibrium will be reached after 2 weeks. The elimination period of aripiprazole, on average, is 75 hours. If you take Abilify for a long time, then its accumulation in the body is possible. Metabolization of the drug occurs in the liver. Meals do not affect the absorption of aripiprazole.
The elimination period of aripiprazole, on average, is 75 hours. If you take Abilify for a long time, then its accumulation in the body is possible. Metabolization of the drug occurs in the liver. Meals do not affect the absorption of aripiprazole.
Indications for use:
The drug is intended for the treatment of schizophrenia, in particular its acute attacks, and for maintenance therapy. It is used for the treatment of manic episodes and maintenance therapy in patients with bipolar I disorder who have recently experienced mixed or manic episodes.
Contraindications:
The drug should not be used if sensitivity to the main component, aripiprazole, or other substances included in the composition is detected. Under 18 years of age, it is not recommended to use it. Caution should be taken when taking the drug for people who have problems with the cardiovascular system (myocardial infarction, coronary heart disease, conduction disorders, heart failure), cerebrovascular diseases in conditions predisposing to hypotension (hypovolemia, dehydration, taking antihypertensive drugs) due to the possibility of developing orthostatic hypotension; patients with convulsive seizures and suffering from those diseases that can cause them; with the risk of hyperthermia, in case of overheating, excessive exercise, the use of anticholinergic drugs, dehydration, since antipsychotics can disrupt thermoregulation; patients at risk of aspiration pneumonia, as there may be a violation of the motor function of the esophagus and aspiration; patients who suffer from obesity and have diabetic patients in the family.
Interaction with other drugs:
h3-blocker of histamine receptors famotidine, which is the cause of a strong inhibition of the secretion of hydrochloric acid in the stomach, has little effect on the chemical transformations of Abilify in the body. Therefore, if it is necessary to combine it with inhibitors of CYP2D6 and CYP3A4, then the dose of the drug should be reduced. If you take 30 mg of the drug with a CYP3A4 inducer, then there will be a decrease of 73% and 68% in the AUC and Cmax of the drug, and its active metabolite dehydroaripiprazole will decrease by 71% and 69% respectively. A similar effect of similar potent inducers of CYP2D6 and CYP3A4 should also be expected. Enzymes: CYP2C19, CYP2C9, CYP2C8, CYP2B6, CYP2A6, CYP1A1, CYP1A2 and CYP2E1 are not involved in the metabolism of aripiprazole in vitro. Accordingly, the interaction of the drug with drugs that can activate these enzymes is unlikely. Also, the simultaneous administration of 30 mg of the drug and valproate or lithium does not significantly affect the chemical transformations of aripiprazole in the body.
Dosage and administration:
Abilify is taken orally, regardless of food. Due to possible memory impairment, it is recommended to monitor the patient's intake of Abilify.
Dosage is determined depending on the nature of the disease, concomitant diseases and ongoing therapy. Patients over 65 years of age, as well as patients with impaired liver and kidney function, dose adjustment is not required.
Dosage for schizophrenia:
Abilify is prescribed at an initial dose of 10-15 mg/day (aripiprazole is taken once a day). Maintenance (average) dose - 15 mg / day. The dose can be adjusted by the physician, in clinical studies, the effectiveness of aripiprazole has been confirmed in the dose range of 10-30 mg / day.
Dosage for manic episodes due to bipolar disorder:
Abilify is prescribed at an initial dose of 15-30 mg / day (take aripiprazole 1 time per day). Dose changes can be made at intervals of 24 hours. The dose can be titrated by a physician, studies have confirmed effectiveness in doses of 15-30 mg / day for a course of 3-12 weeks. The efficacy and safety of doses above 30 mg/day have not been evaluated in clinical studies.
The efficacy and safety of doses above 30 mg/day have not been evaluated in clinical studies.
If patients with bipolar 1 disorder, mixed episodes or manic episodes do not have symptoms during 6 weeks of Abilify (15-30 mg/day), maintenance therapy is considered effective. It is necessary to periodically conduct diagnostics to determine the appropriateness of further maintenance therapy.
Side effects:
During therapy with Abilify tablets, such an undesirable effect on:
- CCC: tachycardia or bradycardia, hypotension (including orthostatic), atrioventricular block, ischemia of the heart muscle, phlebitis, extrasystole, palpitations, increased QT interval, hemorrhage, heart failure, deep vein thrombosis, atrial fibrillation, cardiac arrest . In rare cases: loss of consciousness, cerebral ischemia, atrial flutter, vasovagal syndrome, thrombophlebitis.
- Gastrointestinal tract: change in appetite, difficulty swallowing, caries, flatulence, gastritis, gastroenteritis, gastroesophageal reflux, gingivitis, gastrointestinal bleeding, hemorrhoids, weakness of the anal sphincter, colitis, stomatitis, mucosal ulceration, oral candidiasis, belching, swelling of the tongue, fecaloma, cholelithiasis, cholecystitis, vomiting, stool disorders. In rare cases: increased activity of ALT and AST, pancreatitis, esophagitis, vomiting with blood, bleeding gums, cheilitis, perforation of the gastrointestinal tract wall, liver enlargement, hepatitis.
In rare cases: increased activity of ALT and AST, pancreatitis, esophagitis, vomiting with blood, bleeding gums, cheilitis, perforation of the gastrointestinal tract wall, liver enlargement, hepatitis.
- Musculoskeletal system: rhabdomyolysis, increased activity of creatine phosphokinase, rheumatoid arthritis, tendinitis, arthritis, tendobursitis, myopathy, muscle pain, muscle weakness, muscle cramps and twitching, bone and joint pain.
- CNS: extrapyramidal conditions, dizziness, impaired psychomotor activity, nervousness, depressive states, tremor, hypersalivation, manic and suicidal thoughts, gait disturbances, confusion, hostility, insomnia, akathisia, drowsiness. In rare cases: hallucinations, buccoglossal syndrome, euphoria, decreased reflexes, obsessive thoughts, dystonia, neuroleptic malignant syndrome, paresthesia, decreased memory and concentration, changes in libido, stupor, amnesia, hyperactivity, panic reactions. In addition, the appearance of restless legs syndrome, depersonalization, hyperreflexia, myoclonus, increased sensitivity to stimuli is possible.
- Respiratory system: shortness of breath, bronchospasm, epistaxis, laryngitis, hiccups, pneumonia. In rare cases: cough with blood, increased sputum production, aspiration pneumonia, dryness of the nasal mucosa, hypoxia, pulmonary embolism, respiratory failure, pulmonary edema, apnea.
- Sense organs: earache, conjunctivitis, lacrimation, taste change, otitis media, cataracts, eye pain, frequent blinking, deafness, ocular hemorrhages, diplopia, tinnitus, photophobia.
- Genitourinary system: leukorrhea, urinary disorders, hematuria, cystitis, urinary retention, amenorrhea, dysuria, vaginal bleeding, premature ejaculation, candidiasis, menorrhagia, kidney stones, renal failure, polyuria, nocturia, urinary incontinence. In rare cases: cervicitis, pain in the mammary glands, anorgasmia, galactorrhea, burning sensation in the external genital area, painful erection, gynecomastia, glycosuria, urolithiasis.
Allergic reactions: urticaria, maculopapular rash, eczema, exfoliative dermatitis, pruritus, hyperhidrosis, dry skin, alopecia, acne, vesiculobullous rash, seborrhea, psoriasis, anaphylaxis, angioedema.
Other negative effects: impotence, flu-like syndrome, neck and chest pain, sore throat, bloating, weakness, jaw stiffness, headache, pelvic pain, peripheral edema, swelling of the face, chest tension. In addition, hyperglycemia or hypoglycemia, hyperlipidemia, hyperbilirubinemia, hypercreatininemia, hyperuricemia, hypercholesterolemia, an increase or decrease in serum potassium and sodium levels, and an acid urine sample are possible.
The development of gout, cyanosis, dehydration, thirst, iron deficiency anemia, obesity was also recorded.
Selected adverse events during treatment:
Suicidal attempts:
Suicidal thoughts and attempts are common in patients with psychosis, in the treatment of such patients it is recommended to use the minimum dose of aripiprazole and monitor the patient's condition and behavior. Drug therapy should be carried out simultaneously with psychotherapy.
Tardive dyskinesia:
With an increase in the duration of antipsychotic therapy, the risk of tardive dyskinesia increases. If symptoms of this condition appear, it is necessary to reduce the dose of aripiprazole or stop taking it. After discontinuation of the drug Abilify, there is a possibility of an increase in signs of tardive dyskinesia, in some patients, symptoms appear for the first time after stopping the use of Abilify tablets.
If symptoms of this condition appear, it is necessary to reduce the dose of aripiprazole or stop taking it. After discontinuation of the drug Abilify, there is a possibility of an increase in signs of tardive dyskinesia, in some patients, symptoms appear for the first time after stopping the use of Abilify tablets.
Neuroleptic malignant syndrome:
Patients treated with neuroleptics, including aripiprazole, have reported cases of neuroleptic malignant syndrome, which is accompanied by muscle rigidity, mental disturbances, hyperpyrexia, irregular blood pressure and pulse, as well as sweating, rhabdomyolysis, acute kidney failure and increased activity of creatinine phosphokinase. If the patient has symptoms of neuroleptic malignant syndrome, it is necessary to stop taking antipsychotics.
Hyperglycemia:
Patients treated with atypical antipsychotics have in some cases developed hyperglycemia (which may be associated with ketoacidosis) and hyperosmolar coma (sometimes fatal). A direct relationship between treatment with atypical antipsychotics and a glycemic type disorder has not been clearly established, however, patients who have been diagnosed with diabetes mellitus should monitor their glucose levels. The need to control glucose levels also applies to people at risk of diabetes.
A direct relationship between treatment with atypical antipsychotics and a glycemic type disorder has not been clearly established, however, patients who have been diagnosed with diabetes mellitus should monitor their glucose levels. The need to control glucose levels also applies to people at risk of diabetes.
Special instructions:
Psychosis is characterized by a tendency to suicidal thoughts and attempts to commit them, in this case, treatment with this remedy should be under medical supervision. It should be prescribed in minimal doses, as far as possible in the treatment of the patient. This is necessary in order to reduce the risk of overdose. Long-term therapy with antipsychotics can lead to tardive dyskinesia, which may appear in the future. But, this is a temporary and quickly passing effect. Treatment with antipsychotics, and in particular aripiprazole, can lead to the onset of neuroleptic malignant syndrome. It can be life threatening and manifest itself through mental disorders, muscle rigidity, hyperpyrrhexia, instability of the autonomic nervous system (jumping pressure, irregular pulse, arrhythmia, tachycardia, sweating). In some cases, myoglobinuria, acute renal failure, increased activity of creatine phosphokinase may be observed. If such symptoms occur, then this drug should be discontinued. Patients with diabetes mellitus should be treated with caution. They should check their blood glucose levels regularly. And those patients who are at risk of developing diabetes, because they have family members who suffer from it, or are obese, should check their blood glucose levels before taking aripiprazole, and then do such checks periodically while taking the drug. Patients taking these drugs should be constantly monitored for symptoms of hyperglycemia, attention should be paid to weakness, increased thirst, polyphagia, frequent urination. In addition, the drug affects the ability to control mechanisms and the car.
In some cases, myoglobinuria, acute renal failure, increased activity of creatine phosphokinase may be observed. If such symptoms occur, then this drug should be discontinued. Patients with diabetes mellitus should be treated with caution. They should check their blood glucose levels regularly. And those patients who are at risk of developing diabetes, because they have family members who suffer from it, or are obese, should check their blood glucose levels before taking aripiprazole, and then do such checks periodically while taking the drug. Patients taking these drugs should be constantly monitored for symptoms of hyperglycemia, attention should be paid to weakness, increased thirst, polyphagia, frequent urination. In addition, the drug affects the ability to control mechanisms and the car.
Overdose :
In studies of aripiprazole, there is information about a single dose of 1080 mg, which was accompanied by the development of an overdose and had a favorable outcome.
Patients intoxicated with aripiprazole have experienced drowsiness, vomiting, weakness, and diarrhea. In patients who were hospitalized due to an overdose of the drug Abilify, there were no changes in ECG, laboratory and physiological parameters.
In post-marketing studies, there have been cases of adult patients taking up to 450 mg of aripiprazole, after which tachycardia developed. In children with an overdose (single dose up to 195 mg) develop potentially dangerous effects, including loss of consciousness and extrapyramidal symptoms.
Treatment of Abilify overdose includes symptomatic agents, supportive measures (including maintaining a normal airway, mechanical ventilation, oxygenation).
In the treatment of overdose, it is necessary to take into account clinical symptoms and monitor the function of the cardiovascular system with ECG registration (for the timely detection of rhythm disturbances).
The use of sorbents (in particular activated charcoal) within an hour after taking Abilify tablets orally reduces the peak levels of aripiprazole and AUC by 41% and 51%, respectively.
The effectiveness of hemodialysis in aripiprazole intoxication has not been studied, but theoretically (given the degree of association with albumin and the pattern of excretion) hemodialysis does not lead to a significant decrease in serum levels of this drug.
Shelf life: 3 years
Vacation Conditions: without a prescription
Manufacturer: Bristol-Maires Squib menus (Bristol-Myers Squibb) of the US81, 4602505007001, 4602505006967)
Smart pills as an ethical issue - expert material, Lahta Clinic
The US Food and Drug Administration (FDA) on November 13, 2017 issued a decision that immediately became the focus of attention of the world medical community and became the subject of the widest discussion. The FDA, the most authoritative organization, whose opinion is heeded all over the world, "only" approved the drug Abilify MySite for use in clinical practice. The seemingly ordinary procedure for certification of a medicinal product gave rise to heated discussions and again raised a wave of public interest in such a painful at all times, urgent and acute problem as medical ethics.
The terms “smart pill” (mostly dietary supplements) and “smart case for pills”, as well as several pharmaceutical trademarks registered in the post-Soviet territory using the word “smart”, have nothing to do with that “smart pill "(eng. "smart pill"), which is devoted to this review.
Let's start with the trade name "Abilify MyCite". It is very difficult to translate this game of words-neologisms into Russian: something like “Give capacity ...” or “Bring back opportunities in my case (precedent)”. Previously, the drug "Abilify Maintena" (approx. translation "Source of Opportunities Supporting") has already been approved for use, certified in the Russian Federation under the current registration number LS-001812 dated 11/16/09and sold in pharmacies under the brand name Abilify.
The drug was developed and manufactured by one of the largest Japanese pharmaceutical corporations - Otsuka Pharmaceutical Co., Ltd. In America, Abilify is distributed jointly with Bristol-Myers Squibb (New York), a company in whose name, by the way, a Russian registration certificate has been issued. However, it was Otsuka who submitted the recently approved application for certification to the FDA. This time, she is performing in a somewhat unusual collaboration for a pharmaceutical giant with Proteus Digital Health, a Silicon Valley digital technology developer.
However, it was Otsuka who submitted the recently approved application for certification to the FDA. This time, she is performing in a somewhat unusual collaboration for a pharmaceutical giant with Proteus Digital Health, a Silicon Valley digital technology developer.
Abilify is the trade name for aripiprazole, one of the newer atypical neuroleptics. In turn, antipsychotics are a group of antipsychotic drugs that, since the second half of the 1990s, have become one of the breakthroughs in psychopharmacology, having a number of undeniable advantages over the "traditional" or "typical" first antipsychotics - chlorpromazine and haloperidol. Like other drugs in this group, aripiprazole is used primarily in the treatment of endogenous "major psychoses" - schizophrenia, bipolar disorder and psychotic depression; clinical trials are also underway for the efficacy of aripiprazole in the treatment of alcoholism. This drug has already proven itself quite well in psychiatric practice and, according to the results of comparative studies, surpasses clozapine, olanzapine, risperidone, etc. , released a little earlier, in a number of indicators. The FDA approved the use of aripiprazole in the United States in 2002.
, released a little earlier, in a number of indicators. The FDA approved the use of aripiprazole in the United States in 2002.
And today, after 15 years, Abilify with a new clarifying name is again in the spotlight. For the first time, a mass-produced dosage form containing the so-called. oral or "swallowed" tracer - a microscopic sealed radio frequency emitter that is located inside a standard capsule with the active substance. Once in the intestine, the emitter is activated and sends a signal to a miniature sensor-transmitter, which is attached, like a regular adhesive plaster, to the patient's stomach. This transmitter, in turn, transmits information about the medication to the smartphone. Thus, the patient can keep track of the time of admission and the number of doses he has already taken; in addition, he can provide the doctor and caregivers with access to this information through a dedicated web portal.
Mitchell Mathis, director of psychiatric drugs at the FDA's Center for Drug Research and Evaluation, believes that the ability to monitor prescription medications can be beneficial for some patients with mental disorders.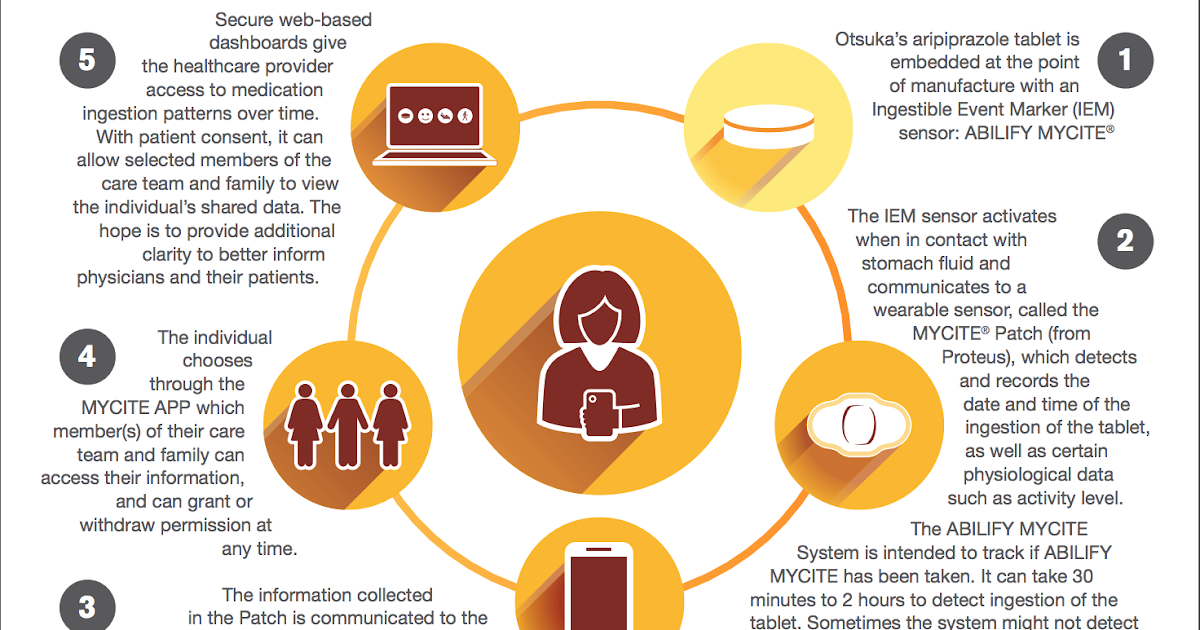 According to M. Mathis - which, apparently, should be considered a reflection of the official position, since they are quoted in a press release - “FDA supports the development and application of new technology, therefore it intends to work with companies in studying all its benefits for patients and doctors."
According to M. Mathis - which, apparently, should be considered a reflection of the official position, since they are quoted in a press release - “FDA supports the development and application of new technology, therefore it intends to work with companies in studying all its benefits for patients and doctors."
So Abilify as such was certified 15 years ago; the oral sensor used in the new dosage form received FDA approval back in 2012. However, the official press release says nothing about the fact that a year and a half ago, an application for certification of a hybrid drug-and-device form was already submitted by Otsuka - and was rejected. The FDA then required companies to provide additional information about the intended use of the product and associated human risk factors, as well as to confirm that the hybrid drug can be safely and effectively used by patients.
In response, Otsuka and Proteus expressed “disappointment with the regulator’s decision,” which did not surprise commentators: a year and a half ago, a lot was written about the fact that the rejection of the application causes serious financial damage to companies, given the funds spent on the upgraded Abilify and fierce competition in the market (formerly only from authorized generic manufacturers of this drug).
Discussions today are dominated by restrained joy at the advancement of new technologies, but there is also some bewilderment. So, commentator Rebecca Robbins notes: forgetfulness and twilight consciousness can accompany those mental disorders for which Abilify is approved for treatment, and patients have to make a lot of effort to take the required medications on time. However, the new drug will come with a warning on the packaging: "Technology has not been proven to help patients take their medications as prescribed."
Immediately following the FDA decision, the journal Anesthesia & Analgesia published the results of a mini-study conducted in Boston on a sample of 15 patients who had suffered fractures and were prescribed opioid analgesics. The study used smart tablets similar to Abilify. The paper's lead author, Dr. Edward Boyer, says the new technology is highly promising, saying it provides a "direct measure" of how many opioids are being taken, which in some cases could be a warning sign of developing tolerance or dependence.
Professor of Psychiatry Tia P. Powell (Albert Einstein College of Medicine, New York) looks at the news from a completely different perspective.
“Unfortunately,” Dr. Powell's article begins, “unfortunately, the first FDA-approved smart pill was a drug used in the treatment of schizophrenia and bipolar disorder. This again raises confusing ethical questions."
Clinical experience and decades of research, it goes on to say, confirm that off-label drug use is a major problem in all branches of medicine. Smart pills can help forgetful people with diabetes, heart problems, and other drug-dependent patients who would like to achieve more and feel better with a prescribed treatment regimen. There is no doubt that, says Prof. Powell that mental illness drastically reduces the quality of life and its duration, and in this regard, drugs can really be useful. It is the WHO who defines mental disorders as the “major contributor” to the global damage from diseases in general. Psychotic episodes are typical of patients who are not taking psychiatrist-prescribed drugs, and such episodes not only further damage the brain, but often endanger both the patients themselves and others. Compliance with prescriptions for patients with mental illness is just as important as for somatic patients. What makes the high-tech version of aripiprazole problematic, however, is that this hybrid drug can easily be used in coercive treatment that ignores the values and preferences of the mentally ill themselves. In general, forced therapy is a long and painful problem in the history of psychiatry. In certain situations, a person with a mental disorder may be subjected, regardless of consent or disagreement, to inpatient or outpatient treatment, sometimes with compulsory medication. Of course, the best medicine in the world won't help, notes Tia P. Powell, if you don't take it. But only at 19In 1970, the US Supreme Court for the first time recognized a legal deficit in people hospitalized against their will.
Psychotic episodes are typical of patients who are not taking psychiatrist-prescribed drugs, and such episodes not only further damage the brain, but often endanger both the patients themselves and others. Compliance with prescriptions for patients with mental illness is just as important as for somatic patients. What makes the high-tech version of aripiprazole problematic, however, is that this hybrid drug can easily be used in coercive treatment that ignores the values and preferences of the mentally ill themselves. In general, forced therapy is a long and painful problem in the history of psychiatry. In certain situations, a person with a mental disorder may be subjected, regardless of consent or disagreement, to inpatient or outpatient treatment, sometimes with compulsory medication. Of course, the best medicine in the world won't help, notes Tia P. Powell, if you don't take it. But only at 19In 1970, the US Supreme Court for the first time recognized a legal deficit in people hospitalized against their will. Both clinicians and patients around the world struggle to strike the right balance between safety and individual rights.
Both clinicians and patients around the world struggle to strike the right balance between safety and individual rights.
A new dosage form is at the very center of this struggle. Manufacturers assure us that patients will consent to such treatment and the use of tracking technology. However, this smart tablet is very attractive for judicial applications. For those patients who are ordered to take drugs by the court, "consent" takes on a very different meaning if it is exchanged for freedom, custody of a child, or a reduced sentence.
I bet, says T. P. Powell, that people will look for ways to outsmart the tracker, while the manufacturers of this expensive drug have gone to great lengths to make it harder to disable the tracker. But I'd rather see an innovation that would serve to build trust and collaboration between doctor and patient.
We need to take a closer look not only at the benefits of Abilify MySite's hybrid drug, the article says, but also at the risks associated with it. It will help some people take their medication as prescribed, but it can also be a high-tech form of psychiatric coercion. If the new drug improves the treatment of "major psychoses", then it is necessary to carefully consider who, in fact, will benefit and who may suffer.
It will help some people take their medication as prescribed, but it can also be a high-tech form of psychiatric coercion. If the new drug improves the treatment of "major psychoses", then it is necessary to carefully consider who, in fact, will benefit and who may suffer.
Abilify MySite has been approved without any indication of its ethical use. “I am convinced,” Professor Powell concludes, “that both clinicians and consumers should be involved in the development of such ethical guidelines. True progress in psychiatry involves a genuine respect for those who struggle with mental illness, and not just the development of new ways to apply treatment to these people.
The publication evoked heated and sometimes diametrically opposed comments from specialists, patients, their relatives and people who are simply not indifferent to the problem.
Robert P.N. Shirin: “Professor Powell seems to be inclined to think of the new drug as something like a Trojan horse in the mouth. I wonder if she sees an ethical problem in DOT (the so-called "Treatment under direct supervision" - approx. Lakhta Clinic), which is a common practice in the fight against tuberculosis?
I wonder if she sees an ethical problem in DOT (the so-called "Treatment under direct supervision" - approx. Lakhta Clinic), which is a common practice in the fight against tuberculosis?
Steven J. Leitner: "The article ignores the fact that, say, long-acting drugs have been around in psychiatry for decades and perform essentially the same function."
Marie Lutz: “There is absolutely no evidence that psychotic episodes “destroy the brain”… Another question—and it is not raised in the article—is who and how will justify compulsory treatment with a neuroleptic with such extensive and serious side effects. effects such as weight gain, tardive dyskinesia, akathisia and reduced life expectancy. In addition, it is doubtful that if atypical antipsychotics can help some people (usually for a short time), then they should necessarily be taken by all other patients. Personally, I believe that the new drug is a "bad faith development" and should be recalled.
Karen D'Angelo: “While I wholeheartedly support a patient's right to choose, as a neighbor of a schizophrenic patient, I also see the consequences of patients not taking their prescribed medications when the judicial system is overly concerned with ensuring compliance (compliance is therapeutic union of a doctor and a patient - approx.