What depression does to the brain
Brain structure alterations in depression: Psychoradiological evidence
1. Jia ZY, Huang XQ, Wu QZ, et al. High‐field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am J Psychiatry. 2010;167:1381‐1390. [PubMed] [Google Scholar]
2. Kong L, Wu F, Tang Y, et al. Frontal‐subcortical volumetric deficits in single episode, medication‐naive depressed patients and the effects of 8 weeks fluoxetine treatment: a VBM‐DARTEL study. PLoS ONE. 2014;9:e79055. [PMC free article] [PubMed] [Google Scholar]
3. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication. JAMA. 2003;289:3095‐3105. [PubMed] [Google Scholar]
4. Eijndhoven P, Wingen GV, Katzenbauer M, et al. Paralimbic cortical thickness in first‐episode depression: evidence for trait‐related differences in mood regulation. Am J Psychiatry. 2013;170:1477‐1486. [PubMed] [Google Scholar]
5. Peng W, Cheng ZQ, Yin L, Jia ZY, Gong QY. Essential brain structural alterations in major depressive disorder: a voxel‐wise meta‐analysis on first episode, medication‐naive patients. J Affect Disord. 2016;199:114‐123. [PubMed] [Google Scholar]
6. Jia Z, Wang Y, Huang X, et al. Impaired frontothalamic circuitry in suicidal patients with depression revealed by diffusion tensor imaging at 3.0 T. J Psychiatry Neurosci. 2014;39:170‐177. [PMC free article] [PubMed] [Google Scholar]
7. Yang XH, Wang Y, Wang DF, et al. White matter microstructural abnormalities and their association with anticipatory anhedonia in depression. Psychiatry Res. 2017;264:29‐34. [PubMed] [Google Scholar]
8. Srivastava S, Bhatia MS, Bhargava SK, Kumari R, Chandra S. A diffusion tensor imaging study using a voxel‐based analysis, region‐of‐interest method to analyze white matter abnormalities in first‐episode, treatment‐naive major depressive disorder. J Neuropsychiatry Clin Neurosci. 2016;28:131‐137. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
9. Chen G, Guo Y, Zhu H, et al. Intrinsic disruption of white matter microarchitecture in first‐episode, drug‐naive major depressive disorder: a voxel‐based meta‐analysis of diffusion tensor imaging. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:179‐187. [PubMed] [Google Scholar]
10. Wei S, Geng H, Jiang X, et al. Amygdala‐prefrontal cortex resting‐state functional connectivity varies with first depressive or manic episode in bipolar disorder. Neurosci Lett. 2017;641:51‐55. [PubMed] [Google Scholar]
11. Shen Z, Jiang L, Yang S, et al. Identify changes of brain regional homogeneity in early and later adult onset patients with first‐episode depression using resting‐state fMRI. PLoS ONE. 2017;12:e0184712. [PMC free article] [PubMed] [Google Scholar]
12. Huang M, Lu S, Yu L, et al. Altered fractional amplitude of low frequency fluctuation associated with cognitive dysfunction in first‐episode drug‐naive major depressive disorder patients. BMC Psychiatry. 2017;17:11. [PMC free article] [PubMed] [Google Scholar]
BMC Psychiatry. 2017;17:11. [PMC free article] [PubMed] [Google Scholar]
13. Zhong X, Pu W, Yao S. Functional alterations of fronto‐limbic circuit and default mode network systems in first‐episode, drug‐naive patients with major depressive disorder: a meta‐analysis of resting‐state fMRI data. J Affect Disord. 2016;206:280‐286. [PubMed] [Google Scholar]
14. van den Heuvel MP, Sporns O. Rich‐club organization of the human connectome. J Neurosci. 2011;31:15775‐15786. [PMC free article] [PubMed] [Google Scholar]
15. Bonelli RM, Cummings JL. Frontal‐subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141‐151. [PMC free article] [PubMed] [Google Scholar]
16. Wang W, Zhao Y, Hu X, et al. Conjoint and dissociated structural and functional abnormalities in first‐episode drug‐naive patients with major depressive disorder: a multimodal meta‐analysis. Sci Rep. 2017;7:10401. [PMC free article] [PubMed] [Google Scholar]
17. Geng H, Wu F, Kong L, et al. Disrupted structural and functional connectivity in prefrontal‐hippocampus circuitry in first‐episode medication‐naive adolescent depression. PLoS ONE. 2016;11:e0148345. [PMC free article] [PubMed] [Google Scholar]
Disrupted structural and functional connectivity in prefrontal‐hippocampus circuitry in first‐episode medication‐naive adolescent depression. PLoS ONE. 2016;11:e0148345. [PMC free article] [PubMed] [Google Scholar]
18. Yeh PH, Zhu H, Nicoletti MA, Hatch JP, Brambilla P, Soares JC. Structural equation modeling and principal component analysis of gray matter volumes in major depressive and bipolar disorders: differences in latent volumetric structure. Psychiatry Res. 2010;184:177‐185. [PMC free article] [PubMed] [Google Scholar]
19. Yi S. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791‐800. [PubMed] [Google Scholar]
20. Gao Q, Zou K, He ZL, Sun XL, Chen HF. Causal connectivity alterations of cortical‐subcortical circuit anchored on reduced hemodynamic response brain regions in first‐episode drug‐naïve major depressive disorder. Sci Rep. 2016;6:21861. [PMC free article] [PubMed] [Google Scholar]
21. Qin J, Liu H, Wei M, et al. Reconfiguration of hub‐level community structure in depressions: a follow‐up study via diffusion tensor imaging. J Affect Disord. 2017;207:305‐312. [PubMed] [Google Scholar]
Qin J, Liu H, Wei M, et al. Reconfiguration of hub‐level community structure in depressions: a follow‐up study via diffusion tensor imaging. J Affect Disord. 2017;207:305‐312. [PubMed] [Google Scholar]
22. Qin J, Wei M, Liu H, et al. Abnormal hubs of white matter networks in the frontal‐parieto circuit contribute to depression discrimination via pattern classification. Magn Reson Imaging. 2014;32:1314‐1320. [PubMed] [Google Scholar]
23. Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in major depressive disorder: a meta‐analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9‐18. [PubMed] [Google Scholar]
24. Lai CH. Hippocampal and subcortical alterations of first‐episode, medication naïve major depressive disorder with panic disorder patients. J Neuropsychiatry Clin Neurosci. 2014;26:142‐149. [PubMed] [Google Scholar]
25. Serra‐Blasco M, Portella MJ, Gómez‐Ansón B, et al. Effects of illness duration and treatment resistance on grey matter abnormalities in major depression. Br J Psychiatry. 2013;202:434‐440. [PubMed] [Google Scholar]
Br J Psychiatry. 2013;202:434‐440. [PubMed] [Google Scholar]
26. Ramezani M, Abolmaesumi P, Tahmaseb A, et al. Fusion analysis of first episode depression: where brain shape deformations meet local composition of tissue. NeuroImage. 2015;7:114‐121. [PMC free article] [PubMed] [Google Scholar]
27. Mayberg HS, Brannan S, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. NeuroReport. 1997;8:1057‐1061. [PubMed] [Google Scholar]
28. Zhang TJ, Wu Q‐Z, Huang XQ, et al. Magnetization transfer imaging reveals the brain deficit in patients with treatment‐refractory depression. J Affect Disord. 2009;117:157‐161. [PubMed] [Google Scholar]
29. Cano M, Martínez‐Zalacaín I, Bernabéu‐Sanz Á, et al. Brain volumetric and metabolic correlates of electroconvulsive therapy for treatment‐resistant depression: a longitudinal neuroimaging study. Transl Psychiatry. 2017;7:e1023. [PMC free article] [PubMed] [Google Scholar]
30. Liu XD, Kakeda S, Watanabe K, et al. Relationship between the cortical thickness and serum cortisol levels in drug‐naive, first‐episode patients with major depressive disorder: a Surface‐based Morphometric Study. Depress Anxiety. 2015;32:702‐708. [PubMed] [Google Scholar]
Relationship between the cortical thickness and serum cortisol levels in drug‐naive, first‐episode patients with major depressive disorder: a Surface‐based Morphometric Study. Depress Anxiety. 2015;32:702‐708. [PubMed] [Google Scholar]
31. Ajilorea O, Narr K, Rosenthal J, et al. Regional cortical gray matter thickness differences associated with type 2 diabetes and major depression. Psychiatry Res. 2010;184:63‐70. [PMC free article] [PubMed] [Google Scholar]
32. Foland‐Ross LC, Sacchet MD, Prasad G, Gilbert B, Thompson PM, Gotlib IH. Cortical thickness predicts the first onset of major depression in adolescence. Int J Dev Neurosci. 2015;46:125‐131. [PMC free article] [PubMed] [Google Scholar]
33. Yang XH, Wang Y, Huang J, et al. Increased prefrontal and parietal cortical thickness does not correlate with anhedonia in patients with untreated first‐episode major depressive disorders. J Neuropsychiatry Clin Neurosci. 2015;234:144‐151. [PubMed] [Google Scholar]
34. Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment‐resistant depression. Int J Neuropsychopharmacol. 2015;18:1‐9. [PMC free article] [PubMed] [Google Scholar]
Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment‐resistant depression. Int J Neuropsychopharmacol. 2015;18:1‐9. [PMC free article] [PubMed] [Google Scholar]
35. Lu Q, Li H, Luo G, et al. Impaired prefrontal‐amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: a dynamic causal modeling study on MEG. Neurosci Lett. 2012;523:125‐130. [PubMed] [Google Scholar]
36. Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci. 2007;1121:546‐561. [PubMed] [Google Scholar]
37. Zhao K, Liu H, Yan R, et al. Altered patterns of association between cortical thickness and subcortical volume in patients with first episode major depressive disorder: a structural MRI study. Psychiatry Res. 2017;260:16‐22. [PubMed] [Google Scholar]
38. Zhang X, Di X, Lei H, et al. Imbalanced spontaneous brain activity in orbitofrontal‐insular circuits in individuals with cognitive vulnerability to depression. J Affect Disord. 2016;198:56‐63. [PubMed] [Google Scholar]
Imbalanced spontaneous brain activity in orbitofrontal‐insular circuits in individuals with cognitive vulnerability to depression. J Affect Disord. 2016;198:56‐63. [PubMed] [Google Scholar]
39. Shen Z, Cheng Y, Yang S, et al. Changes of grey matter volume in first‐episode drug‐naive adult major depressive disorder patients with different age‐onset. Neuroimage Clin. 2016;12:492‐498. [PMC free article] [PubMed] [Google Scholar]
40. Wang Y, Jia Y, Xu G, Ling X, Liu S, Huang L. Frontal white matter biochemical abnormalities in first‐episode, treatment‐naive patients with major depressive disorder: a proton magnetic resonance spectroscopy study. J Affect Disord. 2012;136:620‐626. [PubMed] [Google Scholar]
41. Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta‐analysis of magnetic resonance imaging studies. J Affect Disord. 2011;134:483‐487. [PubMed] [Google Scholar]
42. Zhang X, Yao S, Zhu X, Wang X, Zhu X, Zhong M. Gray matter volume abnormalities in individuals with cognitive vulnerability to depression: a voxel‐based morphometry study. J Affect Disord. 2012;136:443‐452. [PubMed] [Google Scholar]
J Affect Disord. 2012;136:443‐452. [PubMed] [Google Scholar]
43. Choi KS, Riva‐Posse P, Gross RE, Mayberg HS. Mapping the ‘Depression Switch’ during Intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 2015;72:1252‐1260. [PMC free article] [PubMed] [Google Scholar]
44. Fettes P, Schulze L, Downar J. Cortico‐striatal‐thalamic loop circuits of the orbitofrontal cortex: promising therapeutic targets in psychiatric illness. Front Syst Neurosci. 2017;11:25. [PMC free article] [PubMed] [Google Scholar]
45. Lan MJ, Chhetry BT, Liston C, Mann JJ, Dubin M. Transcranial magnetic stimulation of left dorsolateral prefrontal cortex induces brain morphological changes in regions associated with a treatment resistant major depressive episode: an exploratory analysis. Brain Stimul. 2016;9:577‐583. [PMC free article] [PubMed] [Google Scholar]
46. Taber KH, Wen C, Khan A, Hurley RA. The limbic thalamus. J Neuropsychiatry Clin Neurosci. 2004;16:127‐132. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
47. Tekin S, Cummings JL. Frontal‐subcortical neuronal circuits and clinical neuropsychiatry an update. J Psychosom Res. 2002;52:647‐654. [PubMed] [Google Scholar]
48. Lu Y, Liang H, Han D, et al. The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. Neuroimage Clin. 2016;11:658‐666. [PMC free article] [PubMed] [Google Scholar]
49. Zhao YJ, Du MY, Huang XQ, et al. Brain grey matter abnormalities in medication‐free patients with major depressive disorder: a meta‐analysis. Psychol Med. 2014;44:2927‐2937. [PubMed] [Google Scholar]
50. Zhang H, Li L, Wu M, et al. Brain gray matter alterations in first episodes of depression: a meta‐analysis of whole‐brain studies. Neurosci Biobehav Rev. 2016;60:43‐50. [PubMed] [Google Scholar]
51. Zhao K, Liang H, Yan R, et al. Altered patterns of association between cortical thickness and subcortical volume in patients with first episode major depressive disorder: a structural MRI study. Psychiatry Res. 2017;260:16‐22. [PubMed] [Google Scholar]
Psychiatry Res. 2017;260:16‐22. [PubMed] [Google Scholar]
52. Kinoshita M, Nakada M, Okita H, Hamada J, Hayashi Y. Predictive value of fractional anisotropy of the arcuate fasciculus for the functional recovery of language after brain tumor resection: a preliminary study. Clin Neurol Neurosurg. 2014;117:45‐50. [PubMed] [Google Scholar]
53. Chapman CH, Nazem‐Zadeh M, Lee OE, et al. Regional variation in brain white matter diffusion index changes following chemoradiotherapy: a prospective study using tract‐based spatial statistics. PLoS ONE. 2013;8:e57768. [PMC free article] [PubMed] [Google Scholar]
54. Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta‐analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508‐1521. [PubMed] [Google Scholar]
55. Jacobs RH, Barba A, Gowins JR, et al. Decoupling of the amygdala to other salience network regions in adolescent‐onset recurrent major depressive disorder. Psychol Med. 2016;46:1055‐1067. [PMC free article] [PubMed] [Google Scholar]
Psychol Med. 2016;46:1055‐1067. [PMC free article] [PubMed] [Google Scholar]
56. Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late‐life depression. Psychol Med. 2012;42:1203‐1215. [PMC free article] [PubMed] [Google Scholar]
57. Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real‐world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64‐73. [PMC free article] [PubMed] [Google Scholar]
58. Satterthwaite TD, Kable JW, Vandekar L, et al. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015;40:2258‐2268. [PMC free article] [PubMed] [Google Scholar]
59. Koolschijn PCMP, van Haren NEM, Lensvelt‐Mulders GJLM, Hulshoff Pol HE, Kahn RS. Reduced volume of limbic system–affiliated basal ganglia in mood disorders: a meta‐analysis of magnetic resonance imaging studies.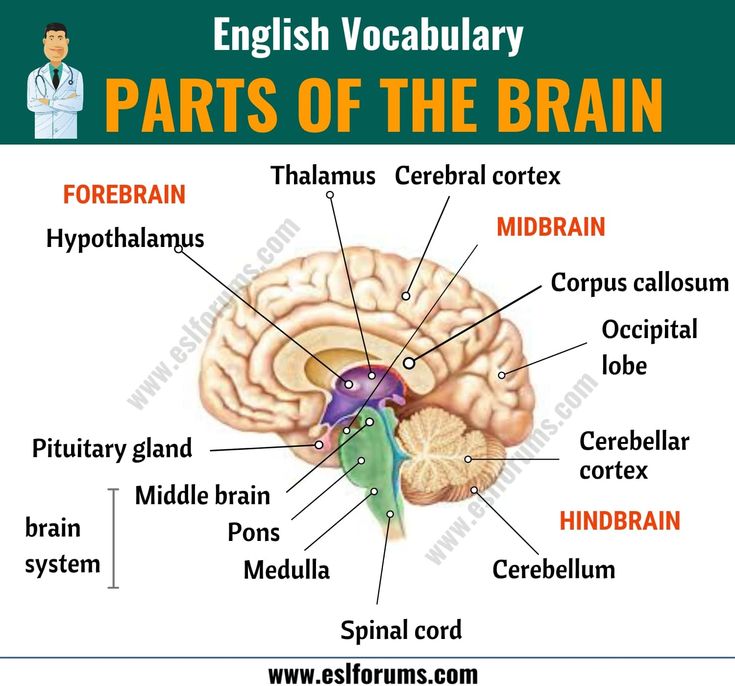 Hum Brain Mapp. 1999;30:3719‐3735. [PMC free article] [PubMed] [Google Scholar]
Hum Brain Mapp. 1999;30:3719‐3735. [PMC free article] [PubMed] [Google Scholar]
60. Koolschijn PC, van Haren NE, Lensvelt‐Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta‐analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719‐3735. [PMC free article] [PubMed] [Google Scholar]
61. Zeki S, Romaya JP. Neural correlates of hate. PLoS ONE. 2008;3:e3556. [PMC free article] [PubMed] [Google Scholar]
62. Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21:806‐812. [PMC free article] [PubMed] [Google Scholar]
63. Stratmann M, Konrad C, Kugel H, et al. Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. PLoS ONE. 2014;9:e102692. [PMC free article] [PubMed] [Google Scholar]
64. Johnston BA, Steele JD, Tolomeo S, Christmas D, Matthews K. Structural MRI‐based predictions in patients with treatment‐refractory depression (TRD). PLoS ONE. 2015;10:e0132958. [PMC free article] [PubMed] [Google Scholar]
Structural MRI‐based predictions in patients with treatment‐refractory depression (TRD). PLoS ONE. 2015;10:e0132958. [PMC free article] [PubMed] [Google Scholar]
65. Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiat. 2006;59:1151‐1159. [PubMed] [Google Scholar]
66. Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlösser RG. Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? NeuroImage. 2011;54:1607‐1614. [PubMed] [Google Scholar]
67. Liu X, Kakeda S, Watanabe K, et al. Relationship between the cortical thickness and serum cortisol levels in drug‐naive, first‐episode patients with major depressive disorder: a surface‐based morphometric study. Depress Anxiety. 2015;32:702‐708. [PubMed] [Google Scholar]
68. Yang XH, Wang Y, Huang J, et al. Increased prefrontal and parietal cortical thickness does not correlate with anhedonia in patients with untreated first‐episode major depressive disorders.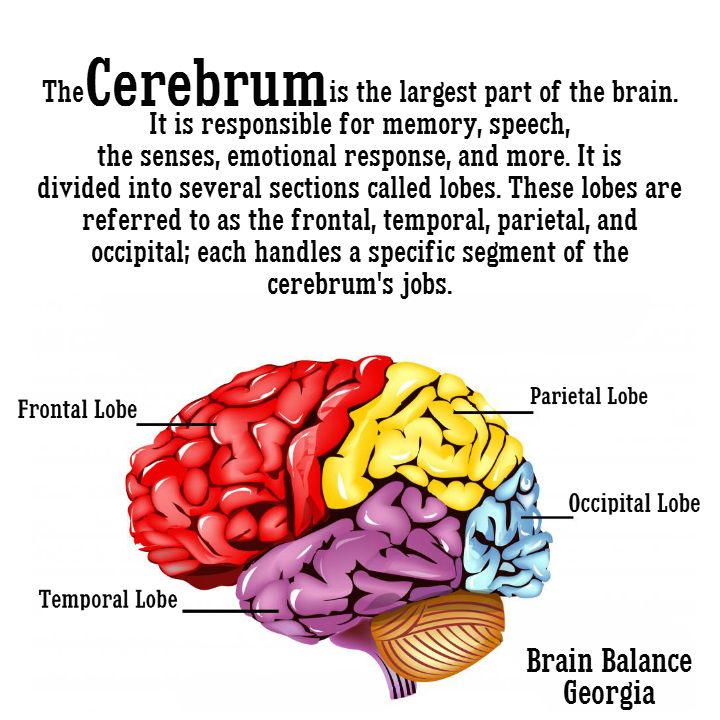 Psychiatry Res. 2015;234:144‐151. [PubMed] [Google Scholar]
Psychiatry Res. 2015;234:144‐151. [PubMed] [Google Scholar]
69. Chen Z, Peng W, Sun H, et al. High‐field magnetic resonance imaging of structural alterations in first‐episode, drug‐naive patients with major depressive disorder. Transl Psychiatry. 2016;6:e942. [PMC free article] [PubMed] [Google Scholar]
70. Liang MJ, Zhou Q, Yang KR, et al. Identify changes of brain regional homogeneity in bipolar disorder and unipolar depression using resting‐state FMRI. PLoS ONE. 2013;8:e79999. [PMC free article] [PubMed] [Google Scholar]
71. Grafton ST, Mazziotta J, Presty S, Friston KJ, Frackowiak RS, Phelps ME. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. J Neurosci. 1992;12:2542‐2548. [PMC free article] [PubMed] [Google Scholar]
72. Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417‐426. [PMC free article] [PubMed] [Google Scholar]
73. Ballmaier M, Narr KL, Toga AW, et al. Hippocampal morphology and distinguishing late‐onset from early‐onset elderly depression. Am J Psychiatry. 2008;165:229‐237. [PMC free article] [PubMed] [Google Scholar]
Ballmaier M, Narr KL, Toga AW, et al. Hippocampal morphology and distinguishing late‐onset from early‐onset elderly depression. Am J Psychiatry. 2008;165:229‐237. [PMC free article] [PubMed] [Google Scholar]
74. Geerlings MI, Gerritsen L. Late‐life depression, hippocampal volumes, and hypothalamic‐pituitary‐adrenal axis regulation: a systematic review and meta‐analysis. Biol Psychiatry. 2017;82:339‐350. [PubMed] [Google Scholar]
75. Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta‐analysis. Am J Psychiatry. 2004;161:598‐607. [PubMed] [Google Scholar]
76. Frodl T, O'Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2013;52:24‐37. [PubMed] [Google Scholar]
77. Yi JH, Brown C, Whitehead G, et al. Glucocorticoids activate a synapse weakening pathway culminating in tau phosphorylation in the hippocampus.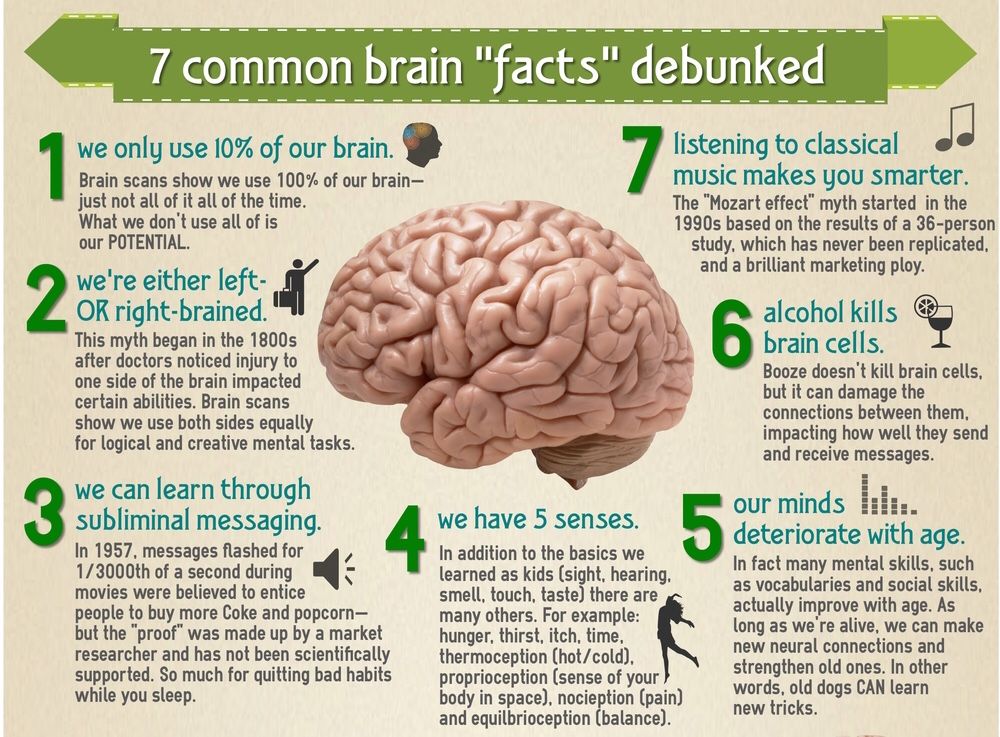 Pharmacol Res. 2017;121:42‐51. [PubMed] [Google Scholar]
Pharmacol Res. 2017;121:42‐51. [PubMed] [Google Scholar]
78. Fu CH, Williams S, Cleare AJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event‐related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877‐889. [PubMed] [Google Scholar]
79. Tendolkar I, Mv B, Oostrom I, et al. Electroconvulsive therapy increases hippocampal and amygdala volume in therapy refractory depression: a longitudinal pilot study. Psychiatry Res. 2013;214:197‐203. [PubMed] [Google Scholar]
80. Eker C, Gonul AS. Volumetric MRI studies of the hippocampus in major depressive disorder: meanings of inconsistency and directions for future research. World J Biol Psychiatry. 2010;11:19‐35. [PubMed] [Google Scholar]
81. Colla M, Kronenberg G, Deuschle M, et al. Hippocampal volume reduction and HPA‐system activity in major depression. J Psychiatr Res. 2007;41:553‐560. [PubMed] [Google Scholar]
82. Shena T, Qiu M, Li C, et al. Altered spontaneous neural activity in first‐episode, unmedicated patients with major depressive disorder. NeuroReport. 2014;25:1302‐1307. [PubMed] [Google Scholar]
Shena T, Qiu M, Li C, et al. Altered spontaneous neural activity in first‐episode, unmedicated patients with major depressive disorder. NeuroReport. 2014;25:1302‐1307. [PubMed] [Google Scholar]
83. Marchand WR. Cortico‐basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Struct Funct. 2010;215:73‐96. [PubMed] [Google Scholar]
84. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357‐381. [PubMed] [Google Scholar]
85. Cummings JL. Frontal‐subcortical circuits and human behavior. Arch Neurol. 1993;50:873‐880. [PubMed] [Google Scholar]
86. Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308‐317. [PubMed] [Google Scholar]
87. Jarbo K, Verstynen TD. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum.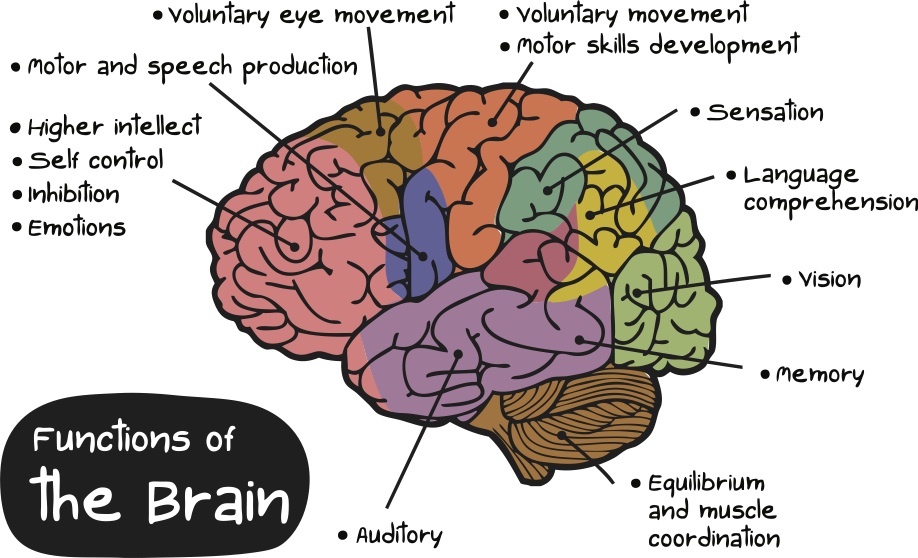 J Neurosci. 2015;35:3865‐3878. [PMC free article] [PubMed] [Google Scholar]
J Neurosci. 2015;35:3865‐3878. [PMC free article] [PubMed] [Google Scholar]
88. Zald DH, McHugo M, Ray KL, Glahn DC, Eickhoff SB, Laird AR. Meta‐analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb Cortex. 2014;24:232‐248. [PMC free article] [PubMed] [Google Scholar]
89. Cavada C, Compañy T, Tejedor J, Cruz‐Rizzolo RJ, Reinoso‐Suárez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220‐242. [PubMed] [Google Scholar]
90. Segarra N, Metastasio A, Ziauddeen H, et al. Abnormal frontostriatal activity during unexpected reward receipt in depression and schizophrenia: relationship to anhedonia. Neuropsychopharmacology. 2016;41:2001‐2010. [PMC free article] [PubMed] [Google Scholar]
91. McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205:667‐677. [PMC free article] [PubMed] [Google Scholar]
92. Wegener I, Geiser F, Alfter S, et al. Changes of explicitly and implicitly measured self‐esteem in the treatment of major depression: evidence for implicit self‐esteem compensation. Compr Psychiatry. 2015;58:57‐67. [PubMed] [Google Scholar]
Wegener I, Geiser F, Alfter S, et al. Changes of explicitly and implicitly measured self‐esteem in the treatment of major depression: evidence for implicit self‐esteem compensation. Compr Psychiatry. 2015;58:57‐67. [PubMed] [Google Scholar]
93. Alexander GE, Crutcher MD, DeLong MR. Basal ganglia‐thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119‐146. [PubMed] [Google Scholar]
94. Zhu X, Helpman L, Papini S, et al. Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress Anxiety. 2017;34:641‐650. [PMC free article] [PubMed] [Google Scholar]
95. Haber SN, Calzavara R. The cortico‐basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78:69‐74. [PMC free article] [PubMed] [Google Scholar]
96. Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545‐574. [PubMed] [Google Scholar]
Annu Rev Psychol. 2002;53:545‐574. [PubMed] [Google Scholar]
97. Davidson CA, Kuroki N, Alvarado JL, Niznikiewicz MA, McCarley RW, Levitt JJ. An MRI study of septi pellucidi in relation to hippocampus volume and fornix integrity in schizophrenia. Schizophr Res. 2012;134:165‐170. [PubMed] [Google Scholar]
98. Lee YJ, Kim S, Gwak AR, et al. Decreased regional gray matter volume in suicide attempters compared to suicide non‐attempters with major depressive disorders. Compr Psychiatry. 2016;67:59‐65. [PubMed] [Google Scholar]
99. Taylor WD, Boyd B, McQuoid DR, Kudra K, Saleh A, MacFall JR. Widespread white matter but focal gray matter alterations in depressed individuals with thoughts of death. Prog Neuropsychopharmacol Biol Psychiatry. 2015;62:22‐28. [PMC free article] [PubMed] [Google Scholar]
100. Sachs‐Ericsson N, Hames JL, Joiner TE, et al. Differences between suicide attempters and non‐attempters in depressed older patients: depression severity, white matter lesions, and cognitive functioning.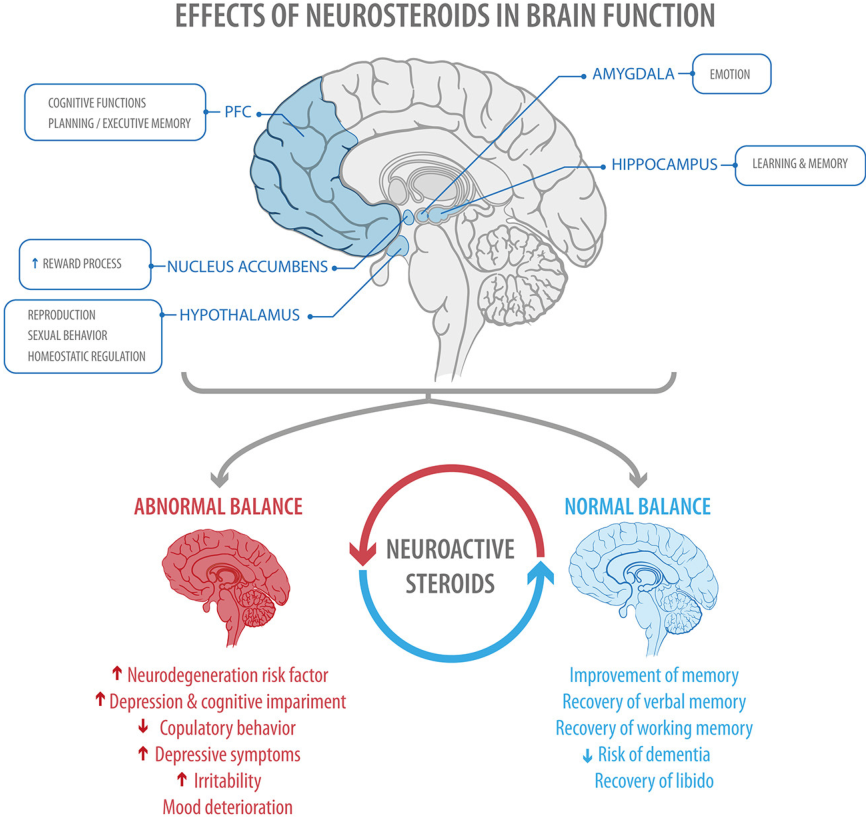 Am J Geriatr Psychiatry. 2014;22:75‐85. [PMC free article] [PubMed] [Google Scholar]
Am J Geriatr Psychiatry. 2014;22:75‐85. [PMC free article] [PubMed] [Google Scholar]
101. Peng HJ, Wu K, Li J, et al. Increased suicide attempts in young depressed patients with abnormal temporal–parietal–limbic gray matter volume. J Affect Disord. 2014;165:69‐73. [PubMed] [Google Scholar]
102. Lai CH, Wu YT. The gray matter alterations in major depressive disorder and panic disorder: putative differences in the pathogenesis. J Affect Disord. 2015;186:1‐6. [PubMed] [Google Scholar]
103. Chen Z, Zhang H, Jia Z, et al. Magnetization transfer imaging of suicidal patients with major depressive disorder. Sci Rep. 2015;5:9670. [PMC free article] [PubMed] [Google Scholar]
104. Colle R, Chuoin M, Cur C, et al. Depressed suicide attempters have smaller hippocampus than depressed patients without suicide attempts. J Psychiatr Res. 2015;61:13‐18. [PubMed] [Google Scholar]
What Does Depression Physically Do to My Brain?
Written by Keri Wiginton
In this Article
- Brain Size
- Brain Inflammation
- Are the Changes Permanent?
- How to Get Help
Depression is more than feeling down. It may physically change your brain.
It may physically change your brain.
This can affect how you think, feel, and act. Experts aren’t sure what causes these changes. They think genetics, stress, and inflammation might play a role.
It’s important to get help for your depression. That’s because repeat episodes seem to damage your brain more and more over time. Early treatment might help you avoid or ease some of the following changes.
Brain Size
There’s some debate about which areas are affected and how much. There’s growing evidence that several parts of the brain shrink in people with depression. Specifically, these areas lose gray matter volume (GMV). That’s tissue with a lot of brain cells. GMV loss seems to be higher in people who have regular or ongoing depression with serious symptoms.
Studies show depression can lower GMV in these areas:
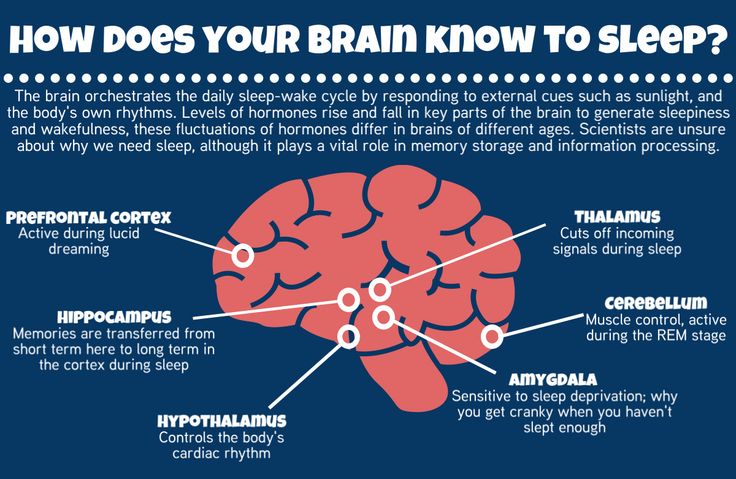
Hippocampus. That part of your brain is important for learning and memory. It connects to other parts of your brain that control emotion and is responsive to stress hormones. That makes it vulnerable to depression.
That makes it vulnerable to depression.
Prefrontal cortex. This area plays a role in your higher-level thinking and planning.
There’s also evidence these parts of your brain get smaller:
- Thalamus
- Caudate nucleus
- Insula
Results are mixed on how depression affects the amygdala. That’s your fear center. Some studies show it gets smaller. Others found that stress and depression might boost its GMV. The more severe the depression, the higher the GMV.
When these areas don’t work the right way, you might have:
- Memory problems
- Trouble thinking clearly
- Guilt or hopelessness
- No motivation
- Sleep or appetite problems
- Anxiety
You might also move or talk slowly, or overreact to negative emotions.
Brain Inflammation
Experts aren’t sure if depression or inflammation comes first. But people who have a major depressive episode have higher levels of translocator proteins. Those are chemicals linked to brain inflammation. Studies show these proteins are even higher in people who’ve had untreated major depressive disorder for 10 years or longer.
Those are chemicals linked to brain inflammation. Studies show these proteins are even higher in people who’ve had untreated major depressive disorder for 10 years or longer.
Uncontrolled brain inflammation can:
- Hurt or kill brain cells
- Prevent new brain cells from growing
- Cause thinking problems
- Speed up brain aging
Are the Changes Permanent?
Scientists are still trying to answer that question. Ongoing depression likely causes long-term changes to the brain, especially in the hippocampus. That might be why depression is so hard to treat in some people. But researchers also found less gray matter volume in people who were diagnosed with lifelong major depressive disorder but hadn’t had depression in years.
While more research is needed, there’s hope that current or new treatments might help reverse or ward off some brain changes.
Here’s what research says about two common depression treatments:
Antidepressants.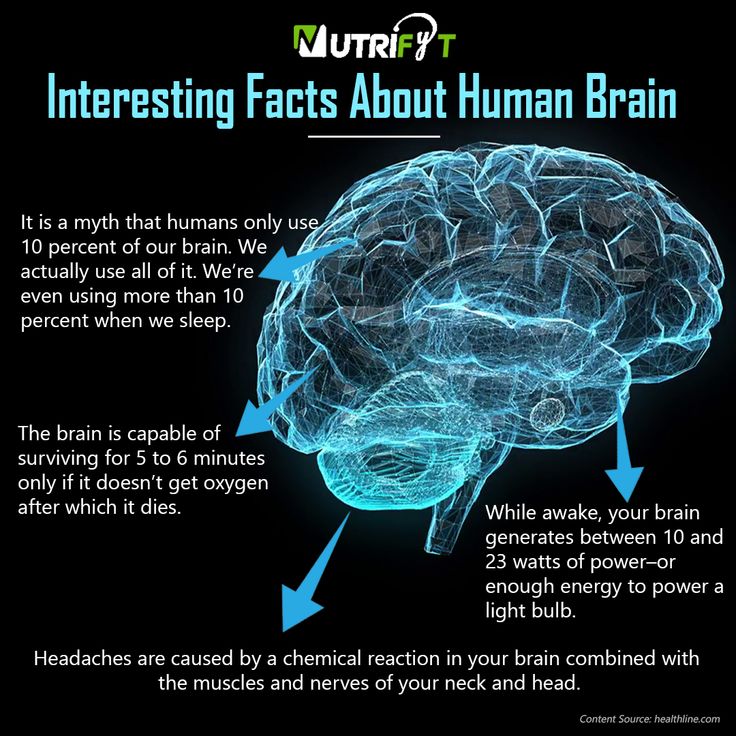 These work on the chemicals in your brain that control stress and emotions. There’s evidence these drugs can help your brain form new connections and lower inflammation.
These work on the chemicals in your brain that control stress and emotions. There’s evidence these drugs can help your brain form new connections and lower inflammation.
Cognitive behavior therapy (CBT). Experts think CBT promotes neuroplasticity. That means you can change your brain in a way that helps your depression.
How to Get Help
Tell your doctor if you have symptoms of depression. They’ll want to rule out other health conditions so they can find you the right treatment. You might need to make some lifestyle changes, take medicine, or talk to a mental health specialist. Some people benefit from a mix of all three.
Some treatments for mild or serious depression include:
- Talk therapy
- Antidepressants
- Short-term use of ketamine
- Brain stimulation
- Exercise
- Meditation
- Healthy diet change
Suicide is a serious symptom of depression. Get help right away if you’re thinking about hurting yourself. You can reach someone at the National Suicide Prevention Lifeline at 1-800-273-8255. They’re available anytime, day or night.
You can reach someone at the National Suicide Prevention Lifeline at 1-800-273-8255. They’re available anytime, day or night.
DEPRESSION IS THE MAIN CAUSE OF DISABILITY IN THE WORLD
This diagnosis is often misdiagnosed. Depression is a disease that has many causes and about which we still do not know much.
Sadness, dark thoughts, low self-esteem, loss of interest or inability to enjoy... Depression is not just a blues, but a real illness. It affects all aspects of daily life and is accompanied by an increased risk of suicide. It can lead to the formation of various addictions, as well as heart disease, diabetes or sexual disorders. nine0006
Many factors are involved in the development of depression. Vulnerability factors are at the basis, for example, if a person was a victim of abuse in childhood. The development of depression is usually preceded by the impact of so-called trigger factors. They can be a breakup in a relationship, the death of a loved one, or financial problems.
Apparently, genetic factors also play a certain role, which makes it possible to speak of hereditary predisposition. Chronic illness, smoking, dependence on alcohol or other psychoactive substances, and even an unbalanced diet can also increase the risk of depression. nine0006
322 million
people living with depression in 2017 1
+ 18.4% 2
Less than half of people with depression receive antidepressant medication. 3
GREAT UNDERSTANDING OF THE CAUSES OF DEPRESSION
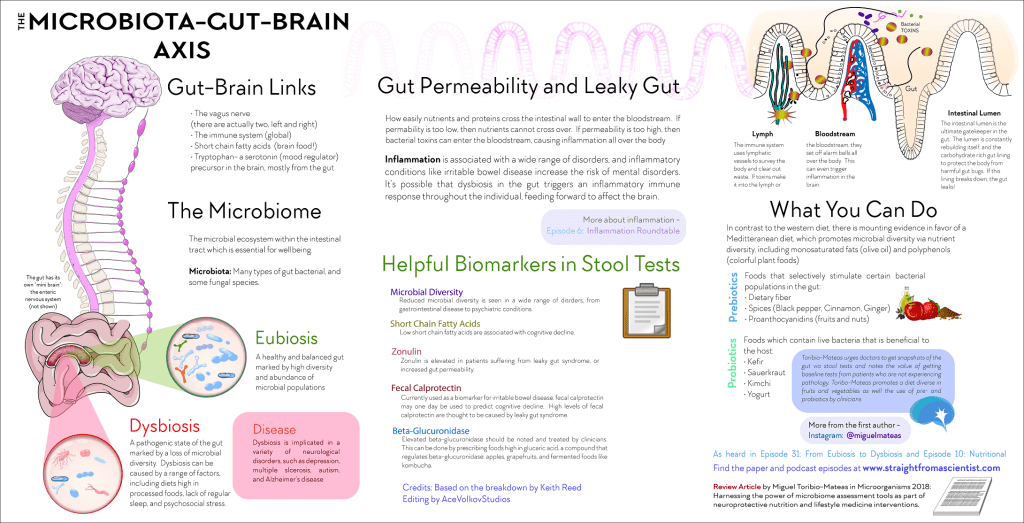
DISTURBANCES IN THE OPERATION OF THE SYSTEM OF NEURO-MEDIATORS
In people with depression, the biochemical processes occurring in the brain are disturbed. This disorder can manifest itself as a deficiency or imbalance in the content of one or more types of neurotransmitters - molecules that are released from the terminal part of the neuron (at the synapse) and act as carriers of chemical signals in the brain.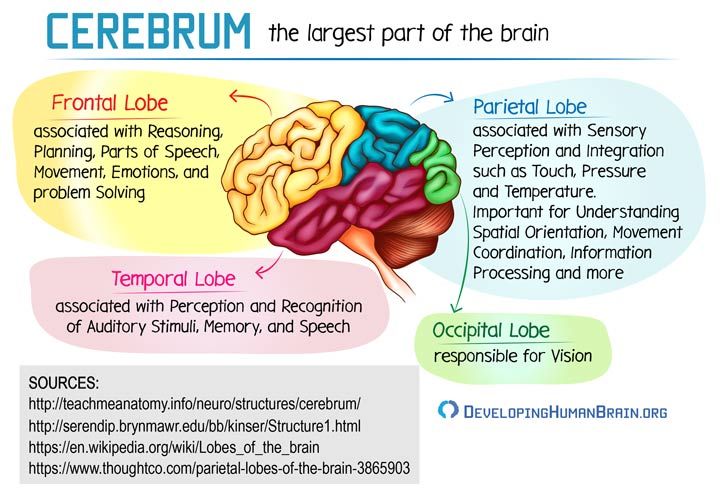 Depression disrupts the balance of three neurotransmitters: serotonin, norepinephrine, and dopamine. They are involved in the regulation of mood and behavior, and their function can be restored with the help of antidepressants. nine0006
Depression disrupts the balance of three neurotransmitters: serotonin, norepinephrine, and dopamine. They are involved in the regulation of mood and behavior, and their function can be restored with the help of antidepressants. nine0006
SYMPTOMS
According to guidelines issued by the World Health Organization and republished by the French health authority (Haute Autorité de Santé) in October 2017, “an episode of depression is characterized by the presence of at least two of the following three main symptoms (see infographic) for two consecutive weeks with a certain degree of severity; they must be different from the patient's previous condition and cause significant distress."
nine0003 Depressive episodes usually resolve after a few weeks or months with treatment or spontaneously. This state is called remission. If subsequent episodes of depression do not recur, recovery is declared, but this rarely happens. In 50–80% of cases, a new episode occurs within the next 5 years. 6 Depression is considered chronic when certain symptoms persist, sometimes less severely, for at least 2 years. nine0006
6 Depression is considered chronic when certain symptoms persist, sometimes less severely, for at least 2 years. nine0006
TREATMENTS
Psychotherapy is recommended regardless of the severity of the depression.
Several types of psychotherapy are used, including supportive, cognitive-behavioral, and analytic-based psychotherapy, as well as psychotherapies based on individual, family, and group sessions.
Relatives and friends invariably play a special role in the treatment of the patient. The patient's expression of his suffering and his acceptance of help are of the utmost importance for successful treatment. nine0006
In addition to psychotherapy, the use of drugs, in particular antidepressants, is most often useful or even necessary.
Antidepressants are recommended for moderate to severe episodes of depression.
There are several classes of antidepressants. Most of them target nerve cells that release serotonin, norepinephrine, or dopamine. Their action is realized through various mechanisms through which the concentration of neurotransmitters increases or the nerve circuits damaged due to depression are restored. nine0006
Their action is realized through various mechanisms through which the concentration of neurotransmitters increases or the nerve circuits damaged due to depression are restored. nine0006
The physician selects the antidepressant that is most appropriate for the patient, based on the patient's symptoms, medical history, past or current conditions and treatment. The effectiveness of antidepressants usually becomes noticeable only after a few weeks.
TWO PHASES OF TREATMENT (THREE IN THE EVENT OF RECURRENCE):
- The acute phase (6 to 12 weeks) is needed to overcome the current episode of depression.
- Consolidation phase (4 to 6 months) aims to reduce the risk of disease recurrence in the short term. nine0072
- Maintenance phase: After three episodes of depression, treatment can be given for several years to prevent relapse.
In most cases, treatment is carried out on an outpatient basis (at the patient's home) under the regular supervision of a healthcare professional. However, sometimes a patient may require emergency care or an episode of depression may be resistant to traditional medications. In this case, hospitalization may be considered. nine0006
However, sometimes a patient may require emergency care or an episode of depression may be resistant to traditional medications. In this case, hospitalization may be considered. nine0006
THE ROLE OF SERVIER
For over 30 years, Servier has provided medical solutions to people who suffer from depression. Recently, the attention of our group has been focused, in particular, on the development of a digital cognitive-behavioral approach.
It combines a cognitive approach, which focuses on correcting the thoughts that keep the patient in a state of emotional decline, and a behavioral approach, which focuses on correcting unacceptable behavior. The goal of therapy is for patients to adopt a new way of thinking and develop optimal behavior. nine0006
RECURRENCE PREVENTION MEASURES
- Incorporate regular, moderate-intensity physical activity into your daily routine
Exercise (walking, running, swimming, cycling) at the recommended frequency of 30-40 minutes 5 times a week.
- Eat a balanced diet
A diet rich in fresh fruits and vegetables, fish and seafood, vegetable oils and whole grains. This type of food is high in essential fatty acids, vitamin B12, selenium, zinc, and iron, a lack of which increases the risk of depression. nine0072 - Discuss your psychological problems without delay
Talking to family, friends or a doctor can help prevent a relapse of depression. In addition, there are communities that provide the necessary assistance to those in need
(1) (2) (3) WHO Report: Depression and other common mental disorders 2017 https://www.who.int/mental_health/management/depression/prevalence_global_health_estimates/en/
(4) http://www.info-depression.fr/spip.php?rubrique16
(5) Léon C, Chan Chee C, du Roscoät E, the Baromètre santé 2017 survey group. La depression en France chez les 18-75 ans: résultats du Baromètre santé 2017. Bulletin épidémiologique hebdomadaire. 2018;(32-33):637-44
2018;(32-33):637-44
https://www.santepubliquefrance.fr/content/download/119666/file/152124_2018-32-33-1.pdf
(6) Website of the French National Inserm Institute for Health and Medical Research, report on depression. nine0006
https://www.inserm.fr/information-en-sante/dossiers-information/depression
NEUROPHYSIOLOGY OF DEPRESSION【 Psychological consultation】
- +38 (067) 448-01-00
- +38 (066) 448-01-00
- [email protected] nine0072
- kpt.center.ua
- Mon.-Fri. 11.00-20.00
- Sat-Sun 10:00-19:00
Sign up for a consultation
Depression is not just sadness or melancholy lasting several days, and not just fatigue. Temporarily giving up after failure is normal. Grieving after the loss of a loved one or the loss of a meaningful relationship is normal. This is not depression, but a normal reaction to difficult circumstances. Clinical depression is a painful condition. Clinical depression is manifested by persistently depressed mood, fatigue, apathy, as well as frequent disturbances in sleep and appetite. The mood can be depressed for several weeks and even months, sometimes for no apparent reason. Fatigue in depression does not go away with rest. The lack of energy can be so strong that it is difficult to perform simple everyday tasks. Cleaning the house or cooking becomes a feat. A therapist can help you understand your condition and understand the difference between fatigue and the depressive or anxious emotions you are experiencing. A therapist can help if you find it difficult to do your daily activities or if you feel overwhelmed. nine0092 In depression, a downward spiral occurs, which is expressed in the fact that the manifestations of depression themselves reinforce the continued existence of depression. For example, on Saturday you are invited to a party, and you think: “something I don’t want to go, and, most likely, it won’t be fun there,” and you decide not to go.
This is not depression, but a normal reaction to difficult circumstances. Clinical depression is a painful condition. Clinical depression is manifested by persistently depressed mood, fatigue, apathy, as well as frequent disturbances in sleep and appetite. The mood can be depressed for several weeks and even months, sometimes for no apparent reason. Fatigue in depression does not go away with rest. The lack of energy can be so strong that it is difficult to perform simple everyday tasks. Cleaning the house or cooking becomes a feat. A therapist can help you understand your condition and understand the difference between fatigue and the depressive or anxious emotions you are experiencing. A therapist can help if you find it difficult to do your daily activities or if you feel overwhelmed. nine0092 In depression, a downward spiral occurs, which is expressed in the fact that the manifestations of depression themselves reinforce the continued existence of depression. For example, on Saturday you are invited to a party, and you think: “something I don’t want to go, and, most likely, it won’t be fun there,” and you decide not to go.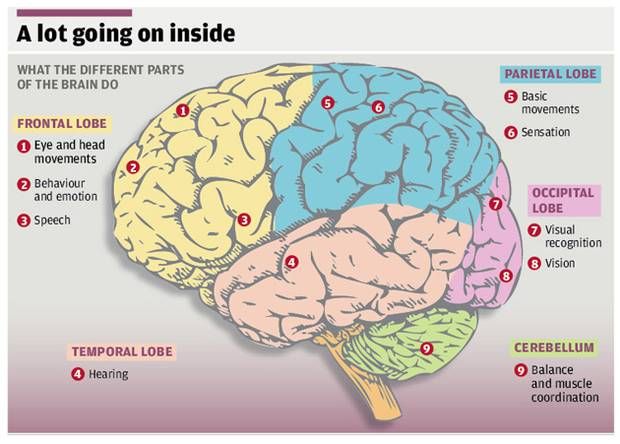 Instead, you, say, lie on the couch for a long time, leafing through the Internet. The next day you sleep for a long time, and when you wake up, you feel overwhelmed. No one calls and you feel lonely, or think that no one is interested in you, or stress other people. These thoughts spoil the mood, and even more so, I don’t want to communicate with anyone. It's not about communicating or not communicating. As a result, such thoughts and feelings lead to the fact that a person stops thinking and doing, even what brings him pleasure. And good mood becomes less. Thus, this downward spiral causes the brain to switch to a negative perception of various situations, and this helps to stabilize the state of depression. nine0092 The therapist can help the person reverse this downward spiral and turn it into an upward spiral. For example, if you don’t even go to a party, but call a friend and talk or meet him in a cafe. This little effort can help you enjoy socializing in a more comfortable environment.
Instead, you, say, lie on the couch for a long time, leafing through the Internet. The next day you sleep for a long time, and when you wake up, you feel overwhelmed. No one calls and you feel lonely, or think that no one is interested in you, or stress other people. These thoughts spoil the mood, and even more so, I don’t want to communicate with anyone. It's not about communicating or not communicating. As a result, such thoughts and feelings lead to the fact that a person stops thinking and doing, even what brings him pleasure. And good mood becomes less. Thus, this downward spiral causes the brain to switch to a negative perception of various situations, and this helps to stabilize the state of depression. nine0092 The therapist can help the person reverse this downward spiral and turn it into an upward spiral. For example, if you don’t even go to a party, but call a friend and talk or meet him in a cafe. This little effort can help you enjoy socializing in a more comfortable environment.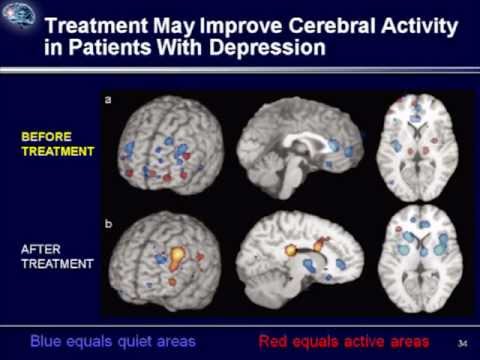 And it is logical that then it is harder to convince yourself of your own loneliness and uselessness. The less sad thoughts appear, the less the mood deteriorates. And most importantly, with the help of simple actions, a small contribution was made to feeling more comfortable. nine0006
And it is logical that then it is harder to convince yourself of your own loneliness and uselessness. The less sad thoughts appear, the less the mood deteriorates. And most importantly, with the help of simple actions, a small contribution was made to feeling more comfortable. nine0006
In depression, not only do negative emotions predominate, but it is also difficult to feel positive emotions. Joy and satisfaction can be difficult to feel, even when a person understands that he should be happy, but he does not feel joy. A psychotherapist can help a person formulate the goals of psychotherapy, one of which is
"I want to feel joy." People, convinced that nothing brings them pleasure, stop looking for pleasant activities. And it is difficult for them to get positive emotions. For example, a person loves to draw and has been drawing as a hobby for some time. Then he stopped drawing, because as a result of a negative assessment of situations, he set high standards for drawings and underestimated the drawing process itself. Thus, the negative sensations take away the beneficial action, and the absence of pleasant activities exacerbates the negative sensation. Considering the situation in a downward spiral is useful, as there are real physiological causes and consequences of the described psychological complexities. nine0092 In clinical depression, the brain works differently than in a healthy state. With depression, the biochemical and physiological processes in the human brain change. In recent decades, changes in the physiology of the brain and the relationship of these changes with the psychological state of a person have been actively studied. Modern research sheds light not only on the formation of individual manifestations of depression, but also on the impact of various treatments on manifestations of depression. The main treatments for depression are antidepressant medication and psychotherapy. nine0092 Several groups of antidepressants are used to treat depression . Serotonin reuptake inhibitors (SSRIs) are usually prescribed for the first episode of depression.
Thus, the negative sensations take away the beneficial action, and the absence of pleasant activities exacerbates the negative sensation. Considering the situation in a downward spiral is useful, as there are real physiological causes and consequences of the described psychological complexities. nine0092 In clinical depression, the brain works differently than in a healthy state. With depression, the biochemical and physiological processes in the human brain change. In recent decades, changes in the physiology of the brain and the relationship of these changes with the psychological state of a person have been actively studied. Modern research sheds light not only on the formation of individual manifestations of depression, but also on the impact of various treatments on manifestations of depression. The main treatments for depression are antidepressant medication and psychotherapy. nine0092 Several groups of antidepressants are used to treat depression . Serotonin reuptake inhibitors (SSRIs) are usually prescribed for the first episode of depression. Second-line drugs include tricyclic antidepressants. Effective types of antidepressants are those developed after 2000, such as serotonin and norepinephrine reuptake inhibitors. Dopamine derivatives and melatonin agonists are often used. Antidepressants help increase energy levels, activity, and improve sleep. Dependence does not develop from antidepressants. With the correct use of antidepressants and the correct termination of their use, the withdrawal syndrome does not develop. Antidepressants are prescribed and stopped under the supervision of a doctor. nine0092 Neurophysiological studies show that the main areas involved in the development of depression are the cerebral cortex, the hippocampus and the amygdala. The cerebral cortex is the most "smart" part of the brain, which is responsible for the analysis of information, complex mental operations such as writing and reading. In depression, a reduced amount of gray matter was found in the cerebral cortex, especially in the frontal (frontal) zone.
Second-line drugs include tricyclic antidepressants. Effective types of antidepressants are those developed after 2000, such as serotonin and norepinephrine reuptake inhibitors. Dopamine derivatives and melatonin agonists are often used. Antidepressants help increase energy levels, activity, and improve sleep. Dependence does not develop from antidepressants. With the correct use of antidepressants and the correct termination of their use, the withdrawal syndrome does not develop. Antidepressants are prescribed and stopped under the supervision of a doctor. nine0092 Neurophysiological studies show that the main areas involved in the development of depression are the cerebral cortex, the hippocampus and the amygdala. The cerebral cortex is the most "smart" part of the brain, which is responsible for the analysis of information, complex mental operations such as writing and reading. In depression, a reduced amount of gray matter was found in the cerebral cortex, especially in the frontal (frontal) zone. This part of the brain is responsible for “must”, for volitional purposeful activity. Also, the frontal cortex is responsible for the interaction between the cortex and subcortical structures. A decrease in the amount of gray matter in the frontal cortex can be manifested by the fact that it becomes difficult for a person to concentrate and consciously remember information. When depressed, it can be difficult to do mental work or study. For example, preparing a report at work can seem like such a big task that it is completely unclear how to approach it. When a person does start writing this report, the work goes worse and slower than expected. In this case, it is easy to give up, stop writing it, which can lead to trouble. In this case, the negative spiral of depression is triggered by the accumulation of problems. With the help of a psychotherapist, a person can reverse this spiral. Discuss that it is useful not to scold yourself for the speed of the work, to appreciate what was nevertheless done.
This part of the brain is responsible for “must”, for volitional purposeful activity. Also, the frontal cortex is responsible for the interaction between the cortex and subcortical structures. A decrease in the amount of gray matter in the frontal cortex can be manifested by the fact that it becomes difficult for a person to concentrate and consciously remember information. When depressed, it can be difficult to do mental work or study. For example, preparing a report at work can seem like such a big task that it is completely unclear how to approach it. When a person does start writing this report, the work goes worse and slower than expected. In this case, it is easy to give up, stop writing it, which can lead to trouble. In this case, the negative spiral of depression is triggered by the accumulation of problems. With the help of a psychotherapist, a person can reverse this spiral. Discuss that it is useful not to scold yourself for the speed of the work, to appreciate what was nevertheless done. This allows you not to give up. That is, to do the work in small steps, regularly investing effort. nine0092 Such changes are accompanied by a steady concentration on any negative information, and may be a manifestation of depression. A reduced amount of gray matter in the cerebral cortex also affects thinking, for example, it can manifest itself as a tendency to negatively evaluate any neutral information (Rude et al., 2002), as well as to more easily recall negative information. Cognitive techniques are used as part of cognitive-behavioral therapy to deal with these changes, which occur due to a decrease in the amount of gray matter in the cerebral cortex. They are aimed at identifying specific assessments of situations or thoughts, and testing them for bias, with the subsequent restructuring of these assessments. nine0092 When considering subcortical structures, the area that most often undergoes changes is the hippocampus, or seahorse (Arnone et al., 2013). It is believed that long-term and biographical memory is concentrated in the hippocampus.
This allows you not to give up. That is, to do the work in small steps, regularly investing effort. nine0092 Such changes are accompanied by a steady concentration on any negative information, and may be a manifestation of depression. A reduced amount of gray matter in the cerebral cortex also affects thinking, for example, it can manifest itself as a tendency to negatively evaluate any neutral information (Rude et al., 2002), as well as to more easily recall negative information. Cognitive techniques are used as part of cognitive-behavioral therapy to deal with these changes, which occur due to a decrease in the amount of gray matter in the cerebral cortex. They are aimed at identifying specific assessments of situations or thoughts, and testing them for bias, with the subsequent restructuring of these assessments. nine0092 When considering subcortical structures, the area that most often undergoes changes is the hippocampus, or seahorse (Arnone et al., 2013). It is believed that long-term and biographical memory is concentrated in the hippocampus. The hippocampus can be compared to a flash drive that contains the story that we tell ourselves about ourselves. It is from this story that we derive deep-seated beliefs about ourselves and about the world. Memories recorded in the hippocampus determine emotional responses to situations that resemble these memories. The hippocampus also plays a role in emotional regulation, specifically in processing emotions, and in responding to positive stimuli. Aboulia, or lack of pleasure in activities that previously brought joy, and lack of motivation for these activities may be associated with a violation of the neurophysiology of the hippocampus. nine0092 Techniques for gaining new emotional experience are used to work with a person's deep beliefs about himself and the world. These techniques are based on the fact that for our brain, in terms of emotional experience, reality and imagination are not so different. To help a person get a new emotional experience, the technique of transcription, or rewriting, is used.
The hippocampus can be compared to a flash drive that contains the story that we tell ourselves about ourselves. It is from this story that we derive deep-seated beliefs about ourselves and about the world. Memories recorded in the hippocampus determine emotional responses to situations that resemble these memories. The hippocampus also plays a role in emotional regulation, specifically in processing emotions, and in responding to positive stimuli. Aboulia, or lack of pleasure in activities that previously brought joy, and lack of motivation for these activities may be associated with a violation of the neurophysiology of the hippocampus. nine0092 Techniques for gaining new emotional experience are used to work with a person's deep beliefs about himself and the world. These techniques are based on the fact that for our brain, in terms of emotional experience, reality and imagination are not so different. To help a person get a new emotional experience, the technique of transcription, or rewriting, is used. This technique involves searching for a memory, more often a childhood one, in which a negative emotional experience was received. After that, the situation in this memory is imagined not as it developed in reality, but as the person would like at the time of rewriting. Gradually, individual memories are "rewritten", and the person receives a new emotional experience. nine0092 Recent studies have shown that the network of nerve cells that is responsible for reward in people with clinical depression is characterized by reduced activation in the limbic part of the brain and increased activation of cells in the cerebral cortex. As a result, a person suffering from depression, analytically, intellectually understands that he can rejoice, but it can be difficult for him to feel joy. Also, intellectually, he can find arguments why it is not worth rejoicing, and emotional sensation cannot balance this argument (Zhang, Chang, Guo, Zhang, & Wang, 2013). nine0092 These features can be balanced using behavioral techniques as part of CBT.
This technique involves searching for a memory, more often a childhood one, in which a negative emotional experience was received. After that, the situation in this memory is imagined not as it developed in reality, but as the person would like at the time of rewriting. Gradually, individual memories are "rewritten", and the person receives a new emotional experience. nine0092 Recent studies have shown that the network of nerve cells that is responsible for reward in people with clinical depression is characterized by reduced activation in the limbic part of the brain and increased activation of cells in the cerebral cortex. As a result, a person suffering from depression, analytically, intellectually understands that he can rejoice, but it can be difficult for him to feel joy. Also, intellectually, he can find arguments why it is not worth rejoicing, and emotional sensation cannot balance this argument (Zhang, Chang, Guo, Zhang, & Wang, 2013). nine0092 These features can be balanced using behavioral techniques as part of CBT. Behavioral techniques help increase the number of enjoyable activities. They allow you to first consciously and systematically monitor the improvement in mood during these activities. This process then becomes automatic and helps reduce depressed mood. These techniques help to use the situation that develops during depression, i.e. when the cerebral cortex is activated strongly, and the limbic (emotional) zone of the brain is activated weakly, to improve the human condition. The cortex, with systematic planning and monitoring of positive activities, fixes how much the mood has improved, and whether there is a feeling of satisfaction, and not how much there is nothing to be happy about. To restore balance between the cortex and the limbic system of the brain, and to improve emotional regulation, the mindfulness technique (or as it is also called mindfulness) is used. Elements of mindfulness practices are used in the course of cognitive behavioral therapy. nine0092 Neuroimaging of the physiology of the brain using functional magnetic resonance imaging (MRI) revealed increased activation of the amygdala in people who suffer from depression compared to people who do not suffer from depression.
Behavioral techniques help increase the number of enjoyable activities. They allow you to first consciously and systematically monitor the improvement in mood during these activities. This process then becomes automatic and helps reduce depressed mood. These techniques help to use the situation that develops during depression, i.e. when the cerebral cortex is activated strongly, and the limbic (emotional) zone of the brain is activated weakly, to improve the human condition. The cortex, with systematic planning and monitoring of positive activities, fixes how much the mood has improved, and whether there is a feeling of satisfaction, and not how much there is nothing to be happy about. To restore balance between the cortex and the limbic system of the brain, and to improve emotional regulation, the mindfulness technique (or as it is also called mindfulness) is used. Elements of mindfulness practices are used in the course of cognitive behavioral therapy. nine0092 Neuroimaging of the physiology of the brain using functional magnetic resonance imaging (MRI) revealed increased activation of the amygdala in people who suffer from depression compared to people who do not suffer from depression.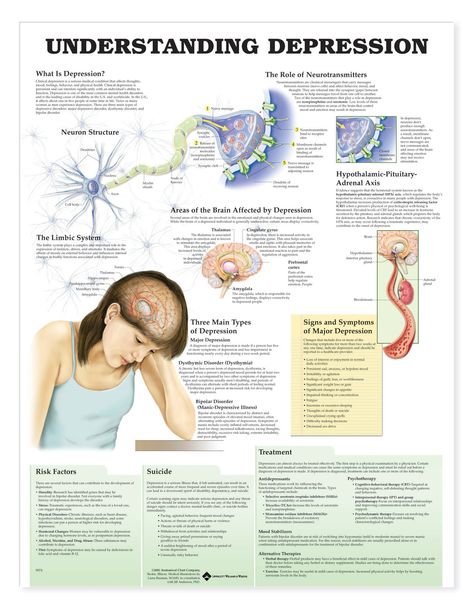 Amygdala can be compared to an antivirus on a computer. It gives a signal when the brain evaluates any stimulus as a threat. Increased amygdala activity is manifested by a tendency to increased anxiety and avoidance behaviors that are perceived as dangerous, which can limit a person's freedom of action. If depression is accompanied by anxiety, there are behavioral techniques specific to dealing with anxiety disorder. nine0092 Not only structural changes in the brain are responsible for the development and maintenance of depression, but also changes in the biochemistry of the brain, for example, anhedonia (the inability to feel pleasure) is associated with disturbances in the dopamine signaling system. Dopamine can be considered the central biochemical mechanism of habit formation. It is dopamine that gives signals that I did it “well”, you can rejoice and feel satisfaction. These cues influence decision-making related to evaluating the balance of effort and the result of actions, as well as the ability to form responses to rewards (Cohen, Najolia, Kim, & Dinzeo, 2012).
Amygdala can be compared to an antivirus on a computer. It gives a signal when the brain evaluates any stimulus as a threat. Increased amygdala activity is manifested by a tendency to increased anxiety and avoidance behaviors that are perceived as dangerous, which can limit a person's freedom of action. If depression is accompanied by anxiety, there are behavioral techniques specific to dealing with anxiety disorder. nine0092 Not only structural changes in the brain are responsible for the development and maintenance of depression, but also changes in the biochemistry of the brain, for example, anhedonia (the inability to feel pleasure) is associated with disturbances in the dopamine signaling system. Dopamine can be considered the central biochemical mechanism of habit formation. It is dopamine that gives signals that I did it “well”, you can rejoice and feel satisfaction. These cues influence decision-making related to evaluating the balance of effort and the result of actions, as well as the ability to form responses to rewards (Cohen, Najolia, Kim, & Dinzeo, 2012). The disruption of dopamine signaling makes it hard for the brain to pass off chemical goodies for previously pleasurable or adaptive behaviors. nine0092 Scientific evidence shows that clinical depression is accompanied by structural, physiological and chemical changes in the brain. These changes are the basis of manifestations of depression. Any high-quality psychotherapy for depression, and in particular, cognitive-behavioral psychotherapy, does not consist only in “talking”, but has certain approaches and techniques with which it can change the functioning of the brain. Psychotherapy, as well as drug treatment, helps improve the state of the brain in clinical depression. nine0006
The disruption of dopamine signaling makes it hard for the brain to pass off chemical goodies for previously pleasurable or adaptive behaviors. nine0092 Scientific evidence shows that clinical depression is accompanied by structural, physiological and chemical changes in the brain. These changes are the basis of manifestations of depression. Any high-quality psychotherapy for depression, and in particular, cognitive-behavioral psychotherapy, does not consist only in “talking”, but has certain approaches and techniques with which it can change the functioning of the brain. Psychotherapy, as well as drug treatment, helps improve the state of the brain in clinical depression. nine0006
References
- Clasen PC, Beevers CG, Mumford JA, Schnyer DM. Cognitive control network connectivity in adolescent women with and without a parental history of depression. developmental cognitive neuroscience. 2014;7:13–22. [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Najolia GM, Kim Y, Dinzeo TJ.
 On the boundaries of blunt affect/alogia across severe mental illness: implications for Research Domain Criteria. Schizophrenia Research. 2012;140:41–45. [PubMed] [Google Scholar]
On the boundaries of blunt affect/alogia across severe mental illness: implications for Research Domain Criteria. Schizophrenia Research. 2012;140:41–45. [PubMed] [Google Scholar] - Cole J, Costafreda SG, McGuffin P, Fu CHY. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. Journal of affective disorders. 2011;134:483–487. [PubMed] [Google Scholar]
- Cook IA, Schrader LM, Degiorgio CM, Miller PR, Maremont ER, Leuchter AF. Trigeminal nerve stimulation in major depressive disorder: acute outcomes in an open pilot study. Epilepsy & behavior: E&B. 2013;28:221–226. [PubMed] [Google Scholar]
- Kerestes R, Ladouceur CD, Meda S, Nathan PJ, Blumberg HP, Maloney K, Phillips ML. Abnormal prefrontal activity subserving attentional control of emotion in remitted
- depressed patients during a working memory task with emotional distracters. psychological medicine. 2012;42:29–40. [PubMed] [Google Scholar]
- Watson LA, Berntsen D, Kuyken W, Watkins ER.
 Involuntary and voluntary autobiographical memory specificity as a function of depression. Journal of Behavior Therapy and Experimental Psychiatry. 2013;44:7–13. [PubMed] [Google Scholar]
Involuntary and voluntary autobiographical memory specificity as a function of depression. Journal of Behavior Therapy and Experimental Psychiatry. 2013;44:7–13. [PubMed] [Google Scholar] - Zeng LL, Shen H, Liu L, Hu D. Unsupervised classification of major depression using functional connectivity MRI. human brain mapping. 2013;35:1630–1641. [PMC free article] [PubMed] [Google Scholar]
- Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. Journal of Affective Disorders. 2013;151:531–539. [PubMed] [Google Scholar]
12/13/2021
Development of tolerance for uncertainty in the treatment of generalized anxiety disorder It is probably difficult to imagine a person who would…
15.