Vyvanse medicine for adhd
Vyvanse® (lisdexamfetamine dimesylate) Uses, Side Effects, and Dosing
MEDICATION GUIDE
(lisdexamfetamine dimesylate)
Capsules and Chewable Tablets, CII
What is the most important information I should know about VYVANSE?
VYVANSE may cause serious side effects, including:
- Abuse and dependence. VYVANSE, other amphetamine containing medicines, and methylphenidate have a high chance for abuse and may cause physical and psychological dependence. Your healthcare provider should check you or your child for signs of abuse and dependence before and during treatment with VYVANSE.
- Tell your healthcare provider if you or your child have ever abused or been dependent on alcohol, prescription medicines, or street drugs.
- Your healthcare provider can tell you more about the differences between physical and psychological dependence and drug addiction.
- Heart-related problems including:
- sudden death, stroke, and heart attack in adults
- sudden death in children who have heart problems or heart defects
- increased blood pressure and heart rate
Your healthcare provider should check you or your child carefully for heart problems before starting treatment with VYVANSE. Tell your healthcare provider if you or your child have any heart problems, heart defects, high blood pressure, or a family history of these problems.
Your healthcare provider should check your or your child’s blood pressure and heart rate regularly during treatment with VYVANSE.
Call your healthcare provider or go to the nearest hospital emergency room right away if you or your child have any signs of heart problems such as chest pain, shortness of breath, or fainting during treatment with VYVANSE.
- Mental (psychiatric) problems, including:
- new or worse behavior and thought problems
- new or worse bipolar illness
- new psychotic symptoms (such as hearing voices, or seeing or believing things that are not real) or new manic symptoms
Tell your healthcare provider about any mental problems you or your child have or about a family history of suicide, bipolar illness, or depression.
Call your healthcare provider right away if you or your child have any new or worsening mental symptoms or problems during treatment with VYVANSE, especially hearing voices, seeing or believing things that are not real, or new manic symptoms.
What Is VYVANSE?
VYVANSE is a central nervous system (CNS) stimulant prescription medicine used for the treatment of:
- Attention Deficit Hyperactivity Disorder (ADHD) in adults and children 6 years of age and older. VYVANSE may help increase attention and decrease impulsiveness and hyperactivity in people with ADHD.
- Moderate to severe binge eating disorder (BED) in adults. VYVANSE may help reduce the number of binge eating days in people with BED.
VYVANSE is not for use in children under 6 years of age with ADHD.
VYVANSE is not for weight loss. It is not known if VYVANSE is safe and effective for the treatment of obesity.
It is not known if VYVANSE is safe and effective for use in children with BED.
VYVANSE is a federally controlled substance (CII) because it contains lisdexamfetamine dimesylate that can be a target for people who abuse prescription medicines or street drugs. Keep VYVANSE in a safe place to protect it from theft. Never give your VYVANSE to anyone else because it may cause death or harm them. Selling or giving away VYVANSE may harm others and is against the law.
Never give your VYVANSE to anyone else because it may cause death or harm them. Selling or giving away VYVANSE may harm others and is against the law.
Do not take VYVANSE if you or your child are:
- allergic to amphetamine products or any of the ingredients in VYVANSE. See the end of this Medication Guide for a complete list of ingredients in VYVANSE.
- taking, or have stopped taking in the last 14 days, a medicine called a Monoamine Oxidase Inhibitor (MAOI).
- being treated with the antibiotic linezolid or intravenous methylene blue.
Before taking VYVANSE, tell your healthcare provider about all medical conditions, including if you or your child:
- have heart problems, heart defects, or high blood pressure
- have mental problems including psychosis, mania, bipolar illness, or depression or have a family history of suicide, bipolar illness, or depression
- have circulation problems in fingers and toes
- are pregnant or plan to become pregnant.
 VYVANSE may harm the unborn baby.
VYVANSE may harm the unborn baby.- There is a pregnancy registry for females who are exposed to VYVANSE during pregnancy. The purpose of the registry is to collect information about the health of females exposed to VYVANSE and their baby. If you or your child becomes pregnant during treatment with VYVANSE, talk to your healthcare provider about registering with the National Pregnancy Registry for Psychostimulants at 1-866-961-2388 or visit online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/adhd-medications/.
- are breastfeeding or plan to breastfeed. VYVANSE passes into breast milk. You should not breastfeed during treatment with VYVANSE. Talk to your healthcare provider about the best way to feed the baby during treatment with VYVANSE.
Tell your healthcare provider about all the medicines that you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
VYVANSE can affect the way other medicines work and other medicines may affect how VYVANSE works. Taking VYVANSE with other medicines can cause serious side effects. Sometimes the doses of other medicines will need to be changed while taking VYVANSE.
Especially tell your healthcare provider if you or your child take:
| selective serotonin reuptake inhibitors (SSRIs) | serotonin norepinephrine reuptake inhibitors (SNRIs) |
| medicines used to treat migraine headaches called triptans | tricyclic antidepressants |
| lithium | fentanyl |
| tramadol | tryptophan |
| buspirone | St. John’s Wort |
Keep a list of all medicines to show your healthcare provider and pharmacist when you get a new medicine. Your healthcare provider will decide if VYVANSE can be taken with other medicines.
Do not start any new medicine during treatment with VYVANSE without talking to your healthcare provider first.
How should VYVANSE be taken?
- Take VYVANSE exactly as prescribed by your healthcare provider.
- Your healthcare provider may change the dose if needed.
- Take VYVANSE 1 time each day in the morning with or without food.
- Your healthcare provider may sometimes stop VYVANSE treatment for a while to check ADHD or BED symptoms.
- VYVANSE comes in capsules or chewable tablets.
Taking VYVANSE Capsules:
- VYVANSE capsules may be swallowed whole.
- If VYVANSE capsules cannot be swallowed whole, the capsule may be opened and the entire contents sprinkled onto yogurt, or poured into water or orange juice.
- Using a spoon, break apart any powder that is stuck together. Stir the VYVANSE powder and yogurt, water, or orange juice until they are completely mixed together.
- Swallow all the yogurt, water, or orange juice mixture right away. Do not store the yogurt, water, or orange juice mixture.

- It is normal to see a filmy coating on the inside of your glass or container after you eat or drink all the VYVANSE mixture.
Taking VYVANSE Chewable Tablets:
- Chew VYVANSE tablets completely before swallowing.
If you or your child take too much VYVANSE, call your healthcare provider or poison control center at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What should I avoid while taking VYVANSE?
Do not drive, operate machinery, or do other dangerous activities until you know how VYVANSE affects you.
What are possible side effects of VYVANSE?
VYVANSE may cause serious side effects, including:
- See "What is the most important information I should know about VYVANSE?"
- Slowing of growth (height and weight) in children. Children should have their height and weight checked often during treatment with VYVANSE.
 VYVANSE treatment may be stopped if your child is not growing or gaining weight.
VYVANSE treatment may be stopped if your child is not growing or gaining weight. - Circulation problems in fingers and toes (Peripheral vasculopathy, including Raynaud’s phenomenon). Signs and symptoms may include:
- Fingers or toes may feel numb, cool, painful
- Fingers or toes may change color from pale, to blue, to red
Tell your healthcare provider if you or your child have numbness, pain, skin color change, or sensitivity to temperature in your fingers or toes.
Call your healthcare provider right away if you or your child have any signs of unexplained wounds appearing on fingers or toes during treatment with VYVANSE.
- Serotonin Syndrome. A potentially life-threatening problem called serotonin syndrome may happen when VYVANSE is taken with certain other medicines. Stop taking VYVANSE and call your healthcare provider or go to the nearest hospital emergency room right away if you or your child develop any of the following signs and symptoms of serotonin syndrome:
| agitation | fast heartbeat |
| flushing | seizures |
| coma | sweating |
| loss of coordination | confusion |
| dizziness | tremors, stiff muscles, or muscle twitching |
| seeing or hearing things that are not real (hallucination) | changes in blood pressure |
| high body temperature (hyperthermia) | nausea, vomiting, diarrhea |
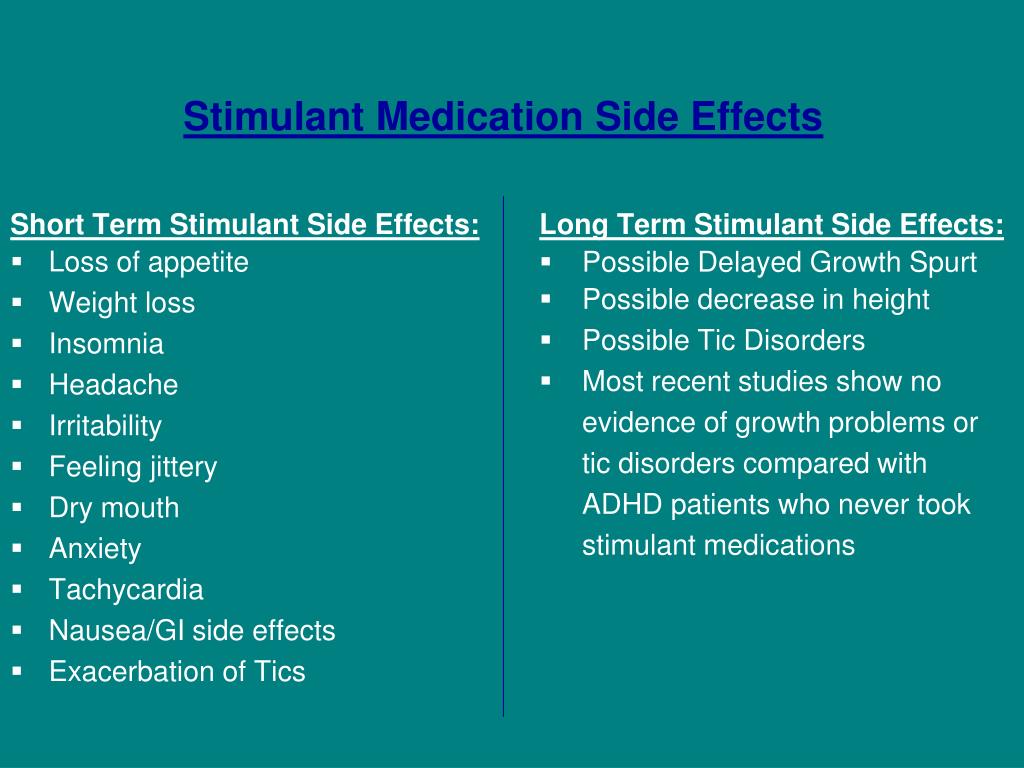
The most common side effects of VYVANSE in children 6 to 17 years old and adults with ADHD include:
| loss of appetite (anorexia) | anxiety |
| decreased appetite | weight loss |
| diarrhea | dizziness |
| dry mouth | irritability |
| trouble sleeping | nausea |
| stomach pain | vomiting |
The most common side effects of VYVANSE in adults with BED include:
| dry mouth | trouble sleeping |
| decreased appetite | increased heart rate |
| constipation | feeling jittery |
| anxiety | |
These are not all the possible side effects of VYVANSE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store VYVANSE?
- Store VYVANSE in a safe place (like a locked cabinet) and in a tightly closed container at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect VYVANSE from light.
- Dispose of remaining, unused, or expired VYVANSE by a medicine take-back program at authorized collection sites such as retail pharmacies, hospital or clinic pharmacies, and law enforcement locations. If no take-back program or authorized collector is available, mix VYVANSE with an undesirable, nontoxic substance such as dirt, cat litter, or used coffee grounds to make it less appealing to children and pets. Place the mixture in a container such as a sealed plastic bag and throw away (discard) VYVANSE in the household trash.
Keep VYVANSE and all medicines out of the reach of children.
General information about the safe and effective use of VYVANSE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VYVANSE for a condition for which it was not prescribed. Do not give VYVANSE to other people, even if they have the same symptoms that you have. It may harm them and it is against the law. You can ask your pharmacist or healthcare provider for information about VYVANSE that is written for health professionals.
What are the ingredients in VYVANSE?
Active Ingredient: lisdexamfetamine dimesylate
Capsule Inactive Ingredients: microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. The capsule shells (imprinted with S489) contain gelatin, titanium dioxide, and one or more of the following: FD&C Red #3, FD&C Yellow #6, FD&C Blue #1, Black Iron Oxide, and Yellow Iron Oxide.
Chewable Tablet Inactive Ingredients: colloidal silicon dioxide, croscarmellose sodium, guar gum, magnesium stearate, mannitol, microcrystalline cellulose, sucralose, artificial strawberry flavor.
Distributed by: Takeda Pharmaceuticals America, Inc., Lexington, MA 02421.
VYVANSE® and the VYVANSE Logo® are registered trademarks of Takeda Pharmaceuticals U.S.A., Inc.
©2022 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
For more information, go to www.vyvanse.com or call 1-877-TAKEDA-7 (1-877-825-3327).
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 10/2021
SPI-0340 Reformatted for US-LIS-1284
What is Vyvanse®? A Prescription Treatment Option for ADHD in Adults
MEDICATION GUIDE
(lisdexamfetamine dimesylate)
Capsules and Chewable Tablets, CII
What is the most important information I should know about VYVANSE?
VYVANSE may cause serious side effects, including:
- Abuse and dependence. VYVANSE, other amphetamine containing medicines, and methylphenidate have a high chance for abuse and may cause physical and psychological dependence.
 Your healthcare provider should check you or your child for signs of abuse and dependence before and during treatment with VYVANSE.
Your healthcare provider should check you or your child for signs of abuse and dependence before and during treatment with VYVANSE.- Tell your healthcare provider if you or your child have ever abused or been dependent on alcohol, prescription medicines, or street drugs.
- Your healthcare provider can tell you more about the differences between physical and psychological dependence and drug addiction.
- Heart-related problems including:
- sudden death, stroke, and heart attack in adults
- sudden death in children who have heart problems or heart defects
- increased blood pressure and heart rate
Your healthcare provider should check you or your child carefully for heart problems before starting treatment with VYVANSE. Tell your healthcare provider if you or your child have any heart problems, heart defects, high blood pressure, or a family history of these problems.
Your healthcare provider should check your or your child’s blood pressure and heart rate regularly during treatment with VYVANSE.
Call your healthcare provider or go to the nearest hospital emergency room right away if you or your child have any signs of heart problems such as chest pain, shortness of breath, or fainting during treatment with VYVANSE.
- Mental (psychiatric) problems, including:
- new or worse behavior and thought problems
- new or worse bipolar illness
- new psychotic symptoms (such as hearing voices, or seeing or believing things that are not real) or new manic symptoms
Tell your healthcare provider about any mental problems you or your child have or about a family history of suicide, bipolar illness, or depression.
Call your healthcare provider right away if you or your child have any new or worsening mental symptoms or problems during treatment with VYVANSE, especially hearing voices, seeing or believing things that are not real, or new manic symptoms.
What Is VYVANSE?
VYVANSE is a central nervous system (CNS) stimulant prescription medicine used for the treatment of:
- Attention Deficit Hyperactivity Disorder (ADHD) in adults and children 6 years of age and older.
 VYVANSE may help increase attention and decrease impulsiveness and hyperactivity in people with ADHD.
VYVANSE may help increase attention and decrease impulsiveness and hyperactivity in people with ADHD. - Moderate to severe binge eating disorder (BED) in adults. VYVANSE may help reduce the number of binge eating days in people with BED.
VYVANSE is not for use in children under 6 years of age with ADHD.
VYVANSE is not for weight loss. It is not known if VYVANSE is safe and effective for the treatment of obesity.
It is not known if VYVANSE is safe and effective for use in children with BED.
VYVANSE is a federally controlled substance (CII) because it contains lisdexamfetamine dimesylate that can be a target for people who abuse prescription medicines or street drugs. Keep VYVANSE in a safe place to protect it from theft. Never give your VYVANSE to anyone else because it may cause death or harm them. Selling or giving away VYVANSE may harm others and is against the law.
Do not take VYVANSE if you or your child are:
- allergic to amphetamine products or any of the ingredients in VYVANSE.
 See the end of this Medication Guide for a complete list of ingredients in VYVANSE.
See the end of this Medication Guide for a complete list of ingredients in VYVANSE. - taking, or have stopped taking in the last 14 days, a medicine called a Monoamine Oxidase Inhibitor (MAOI).
- being treated with the antibiotic linezolid or intravenous methylene blue.
Before taking VYVANSE, tell your healthcare provider about all medical conditions, including if you or your child:
- have heart problems, heart defects, or high blood pressure
- have mental problems including psychosis, mania, bipolar illness, or depression or have a family history of suicide, bipolar illness, or depression
- have circulation problems in fingers and toes
- are pregnant or plan to become pregnant. VYVANSE may harm the unborn baby.
- There is a pregnancy registry for females who are exposed to VYVANSE during pregnancy. The purpose of the registry is to collect information about the health of females exposed to VYVANSE and their baby. If you or your child becomes pregnant during treatment with VYVANSE, talk to your healthcare provider about registering with the National Pregnancy Registry for Psychostimulants at 1-866-961-2388 or visit online at https://womensmentalhealth.
 org/clinical-and-research-programs/pregnancyregistry/adhd-medications/.
org/clinical-and-research-programs/pregnancyregistry/adhd-medications/.
- There is a pregnancy registry for females who are exposed to VYVANSE during pregnancy. The purpose of the registry is to collect information about the health of females exposed to VYVANSE and their baby. If you or your child becomes pregnant during treatment with VYVANSE, talk to your healthcare provider about registering with the National Pregnancy Registry for Psychostimulants at 1-866-961-2388 or visit online at https://womensmentalhealth.
- are breastfeeding or plan to breastfeed. VYVANSE passes into breast milk. You should not breastfeed during treatment with VYVANSE. Talk to your healthcare provider about the best way to feed the baby during treatment with VYVANSE.
Tell your healthcare provider about all the medicines that you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
VYVANSE can affect the way other medicines work and other medicines may affect how VYVANSE works. Taking VYVANSE with other medicines can cause serious side effects. Sometimes the doses of other medicines will need to be changed while taking VYVANSE.
Especially tell your healthcare provider if you or your child take:
| selective serotonin reuptake inhibitors (SSRIs) | serotonin norepinephrine reuptake inhibitors (SNRIs) |
| medicines used to treat migraine headaches called triptans | tricyclic antidepressants |
| lithium | fentanyl |
| tramadol | tryptophan |
| buspirone | St. John’s Wort John’s Wort |
Keep a list of all medicines to show your healthcare provider and pharmacist when you get a new medicine. Your healthcare provider will decide if VYVANSE can be taken with other medicines.
Do not start any new medicine during treatment with VYVANSE without talking to your healthcare provider first.
How should VYVANSE be taken?
- Take VYVANSE exactly as prescribed by your healthcare provider.
- Your healthcare provider may change the dose if needed.
- Take VYVANSE 1 time each day in the morning with or without food.
- Your healthcare provider may sometimes stop VYVANSE treatment for a while to check ADHD or BED symptoms.
- VYVANSE comes in capsules or chewable tablets.
Taking VYVANSE Capsules:
- VYVANSE capsules may be swallowed whole.
- If VYVANSE capsules cannot be swallowed whole, the capsule may be opened and the entire contents sprinkled onto yogurt, or poured into water or orange juice.
- Using a spoon, break apart any powder that is stuck together. Stir the VYVANSE powder and yogurt, water, or orange juice until they are completely mixed together.
- Swallow all the yogurt, water, or orange juice mixture right away. Do not store the yogurt, water, or orange juice mixture.
- It is normal to see a filmy coating on the inside of your glass or container after you eat or drink all the VYVANSE mixture.
Taking VYVANSE Chewable Tablets:
- Chew VYVANSE tablets completely before swallowing.
If you or your child take too much VYVANSE, call your healthcare provider or poison control center at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What should I avoid while taking VYVANSE?
Do not drive, operate machinery, or do other dangerous activities until you know how VYVANSE affects you.
What are possible side effects of VYVANSE?
VYVANSE may cause serious side effects, including:
- See "What is the most important information I should know about VYVANSE?"
- Slowing of growth (height and weight) in children.
 Children should have their height and weight checked often during treatment with VYVANSE. VYVANSE treatment may be stopped if your child is not growing or gaining weight.
Children should have their height and weight checked often during treatment with VYVANSE. VYVANSE treatment may be stopped if your child is not growing or gaining weight. - Circulation problems in fingers and toes (Peripheral vasculopathy, including Raynaud’s phenomenon). Signs and symptoms may include:
- Fingers or toes may feel numb, cool, painful
- Fingers or toes may change color from pale, to blue, to red
Tell your healthcare provider if you or your child have numbness, pain, skin color change, or sensitivity to temperature in your fingers or toes.
Call your healthcare provider right away if you or your child have any signs of unexplained wounds appearing on fingers or toes during treatment with VYVANSE.
- Serotonin Syndrome. A potentially life-threatening problem called serotonin syndrome may happen when VYVANSE is taken with certain other medicines. Stop taking VYVANSE and call your healthcare provider or go to the nearest hospital emergency room right away if you or your child develop any of the following signs and symptoms of serotonin syndrome:
| agitation | fast heartbeat |
| flushing | seizures |
| coma | sweating |
| loss of coordination | confusion |
| dizziness | tremors, stiff muscles, or muscle twitching |
| seeing or hearing things that are not real (hallucination) | changes in blood pressure |
| high body temperature (hyperthermia) | nausea, vomiting, diarrhea |
The most common side effects of VYVANSE in children 6 to 17 years old and adults with ADHD include:
| loss of appetite (anorexia) | anxiety |
| decreased appetite | weight loss |
| diarrhea | dizziness |
| dry mouth | irritability |
| trouble sleeping | nausea |
| stomach pain | vomiting |
The most common side effects of VYVANSE in adults with BED include:
| dry mouth | trouble sleeping |
| decreased appetite | increased heart rate |
| constipation | feeling jittery |
| anxiety | |
These are not all the possible side effects of VYVANSE.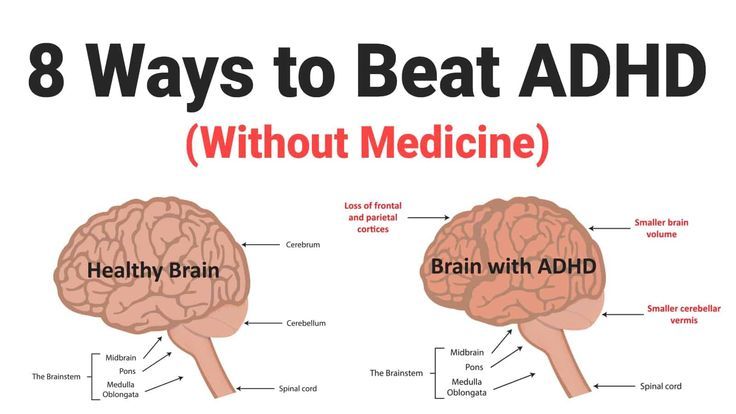
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store VYVANSE?
- Store VYVANSE in a safe place (like a locked cabinet) and in a tightly closed container at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect VYVANSE from light.
- Dispose of remaining, unused, or expired VYVANSE by a medicine take-back program at authorized collection sites such as retail pharmacies, hospital or clinic pharmacies, and law enforcement locations. If no take-back program or authorized collector is available, mix VYVANSE with an undesirable, nontoxic substance such as dirt, cat litter, or used coffee grounds to make it less appealing to children and pets. Place the mixture in a container such as a sealed plastic bag and throw away (discard) VYVANSE in the household trash.
Keep VYVANSE and all medicines out of the reach of children.
General information about the safe and effective use of VYVANSE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VYVANSE for a condition for which it was not prescribed. Do not give VYVANSE to other people, even if they have the same symptoms that you have. It may harm them and it is against the law. You can ask your pharmacist or healthcare provider for information about VYVANSE that is written for health professionals.
What are the ingredients in VYVANSE?
Active Ingredient: lisdexamfetamine dimesylate
Capsule Inactive Ingredients: microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. The capsule shells (imprinted with S489) contain gelatin, titanium dioxide, and one or more of the following: FD&C Red #3, FD&C Yellow #6, FD&C Blue #1, Black Iron Oxide, and Yellow Iron Oxide.
Chewable Tablet Inactive Ingredients: colloidal silicon dioxide, croscarmellose sodium, guar gum, magnesium stearate, mannitol, microcrystalline cellulose, sucralose, artificial strawberry flavor.
Distributed by: Takeda Pharmaceuticals America, Inc., Lexington, MA 02421.
VYVANSE® and the VYVANSE Logo® are registered trademarks of Takeda Pharmaceuticals U.S.A., Inc.
©2022 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
For more information, go to www.vyvanse.com or call 1-877-TAKEDA-7 (1-877-825-3327).
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 10/2021
SPI-0340 Reformatted for US-LIS-1284
Active Stimulation Drug makers got Americans hooked on amphetamines. And they made billions on this: Markets: Economics: Lenta.ru
The United States is facing an epidemic of attention deficit hyperactivity disorder (ADHD) - the number of patients is growing by leaps and bounds. However, not all experts and doctors are confident in the naturalness of this growth - many believe that these are the machinations of pharmaceutical giants who are lobbying for their drugs against ADHD. Parents are convinced of the illness of their children and the urgent need to take stimulants, without which their life is doomed. Some doctors are ready to make a difficult diagnosis based on poor grades at school. At the same time, there is also a significant increase in ADHD patients among adults. An epidemic of attention deficit or an attempt to patch up deficits in the budgets of pharmaceutical giants - in the material "Lenta.ru". nine0003
Parents are convinced of the illness of their children and the urgent need to take stimulants, without which their life is doomed. Some doctors are ready to make a difficult diagnosis based on poor grades at school. At the same time, there is also a significant increase in ADHD patients among adults. An epidemic of attention deficit or an attempt to patch up deficits in the budgets of pharmaceutical giants - in the material "Lenta.ru". nine0003
Attention deficit hyperactivity disorder (ADHD) is a neurological-behavioral developmental disorder. Distinctive signs of ADHD: inattention (a person is not able to pay attention to details, cannot follow instructions for a long time, has difficulty completing a task on his own, often loses things), hyperactivity (an excited state, inability to sit still for a long time, uncontrolled movements are often observed, talkativeness) , aggressive behavior and impulsivity. The disorder causes difficulties in learning and social interaction. nine0003
Millions of people are being diagnosed with Attention Deficit Hyperactivity Disorder (ADHD). Without treatment, living with this disorder means many problems, including financial ones. In 2017, a study was published in the scientific journal PLOS One showing a strong link between ADHD and high-interest loans, in particular from pawnshops, late payments, being late, and constantly changing jobs. This is because people with ADHD, due to their cognitive impairment, respond quickly to short-term stimuli. They are characterized by impulsive behavior that makes them make hasty decisions, this is not a personal choice, but problems caused by the peculiarities of the brain. nine0003
Without treatment, living with this disorder means many problems, including financial ones. In 2017, a study was published in the scientific journal PLOS One showing a strong link between ADHD and high-interest loans, in particular from pawnshops, late payments, being late, and constantly changing jobs. This is because people with ADHD, due to their cognitive impairment, respond quickly to short-term stimuli. They are characterized by impulsive behavior that makes them make hasty decisions, this is not a personal choice, but problems caused by the peculiarities of the brain. nine0003
Photo: Shutterstock
According to the University of Iowa, released in 2018, the number of people with ADHD is growing rapidly. In 2016, 10.2 percent of children aged 4 to 17 received this diagnosis in the United States, compared to 6.1 percent in 1997-1998. In the 1970s, ADHD was considered a childhood disease that would go away with time. Later it turned out that the symptoms persist into adulthood, moreover, ADHD can manifest itself quite late. According to a study by King's College London, about 70 percent of those who were diagnosed after the age of 18 did not have the corresponding symptoms in childhood. “ADHD occurs in about 4 percent of adults, but only a few of them receive medical attention,” says study author Jessica Agnew-Blais. Many people diagnosed with ADHD in adulthood admit to living in crisis for decades, changing one job after another until they were diagnosed, someone managed to get the necessary treatment only after a suicide attempt. nine0003
According to a study by King's College London, about 70 percent of those who were diagnosed after the age of 18 did not have the corresponding symptoms in childhood. “ADHD occurs in about 4 percent of adults, but only a few of them receive medical attention,” says study author Jessica Agnew-Blais. Many people diagnosed with ADHD in adulthood admit to living in crisis for decades, changing one job after another until they were diagnosed, someone managed to get the necessary treatment only after a suicide attempt. nine0003
Related materials:
The spread of ADHD has reached such proportions that it is already a serious burden on the economy. The ADHD Research Center of Canada (CADDAC) estimates that the country loses between $6 billion and $11 billion annually due to lost productivity in the workplace associated with attention deficit disorder. Among people with ADHD there are also quite successful businessmen, but most of them cannot get a job, often change jobs, occupy the lowest paid positions. All this means additional costs for the state, such people are more dependent on the social security system and pay less taxes. nine0003
All this means additional costs for the state, such people are more dependent on the social security system and pay less taxes. nine0003
New market
Therapy can correct the disorder. Most of those who are diagnosed in adulthood admit that after they were able to determine the cause of the problems and prescribe treatment, things improved and they began to lead a full life. The recognition of ADHD has created a huge market for pharmaceutical companies. Once proven that ADHD can manifest into adulthood, “new adults,” who had never been diagnosed before, were the fastest growing segment of the market, as Angus Russell, then CEO of the pharmaceutical company Shire, acknowledged in 2011. According to medical analytics company IMS Health in 2012 for people aged 20 to 3916 million prescriptions for ADHD medications have been filled in the past year.
Photo: Benjamin Vincent Kasapoglu / Wikimedia
Central nervous system stimulants are mainly used for treatment, primarily Ritalin, Adderall, Concerta, Focalin and Vyvanse. Under these trademarks, compounds of amphetamine and methylphenidate are sold, in Russia the circulation of these psychotropic substances is prohibited. According to IMS Health, sales of stimulants were $4 billion in 2007 and by 2012 had risen to $10.5 billion. Between 2012 and 2015, the market reached $11.2 billion. It is predicted that by 2020 it will be about 17.5 billion dollars. Shire has been the market leader in ADHD medications for over 20 years. The company managed to become number one after 19In 1997, it acquired Richwood Pharmaceutical for $186 million, which developed the most popular ADHD treatment, Adderall (an amphetamine-based stimulant). Shire also owns Vyvanse and a number of other brands. The company's ADHD drug divisions are worth about $8.5 billion.
Under these trademarks, compounds of amphetamine and methylphenidate are sold, in Russia the circulation of these psychotropic substances is prohibited. According to IMS Health, sales of stimulants were $4 billion in 2007 and by 2012 had risen to $10.5 billion. Between 2012 and 2015, the market reached $11.2 billion. It is predicted that by 2020 it will be about 17.5 billion dollars. Shire has been the market leader in ADHD medications for over 20 years. The company managed to become number one after 19In 1997, it acquired Richwood Pharmaceutical for $186 million, which developed the most popular ADHD treatment, Adderall (an amphetamine-based stimulant). Shire also owns Vyvanse and a number of other brands. The company's ADHD drug divisions are worth about $8.5 billion.
Prescription nuclear bombs
Scientists don't have a conclusive answer yet as to why the number of people with ADHD is on the rise. Some suggest that the problem is in modern gadgets and digital media: the constant change of bright pictures makes you switch your attention all the time, which can eventually turn into a serious disorder. This version is supported by a two-year study by scientists from the University of Southern California. Dr. Wei Bao from the University of Iowa, whose work has led to talk about the "new epidemic of ADHD", he himself believes that it is too early to draw any conclusions, perhaps people have not become sick more often, just doctors have learned to better identify symptoms. nine0003
This version is supported by a two-year study by scientists from the University of Southern California. Dr. Wei Bao from the University of Iowa, whose work has led to talk about the "new epidemic of ADHD", he himself believes that it is too early to draw any conclusions, perhaps people have not become sick more often, just doctors have learned to better identify symptoms. nine0003
Related materials:
Another point of view is that under the pressure of the pharmaceutical industry, doctors began to make this diagnosis too often. Dr. Keith Conners pioneered the current practice of treating childhood disorders with stimulants. It was he who in the early 1960s at Johns Hopkins University conducted the first official tests on the effects of stimulants (specifically methylphenidate) on children. The studies were paid for by Ritalin manufacturer CIBA. The results were overwhelming: children's academic performance skyrocketed, and Conners became one of the leading advocates for the use of stimulants to treat ADHD. He worked with all the major manufacturers, but then abruptly changed his position and began to talk about a wave of misdiagnosis of ADHD, which reached the level of a "national disaster." nine0003
He worked with all the major manufacturers, but then abruptly changed his position and began to talk about a wave of misdiagnosis of ADHD, which reached the level of a "national disaster." nine0003
Keith Conners
Frame: MHS Assessments - Clinical and Education / YouTube
Conners has repeatedly stated that under pressure from the pharmaceutical giants, the signs of classic ADHD have been unnecessarily expanded. Longtime owner of Richwood Pharmaceutical and the Adderall brand, Roger Griggs, now says he is vehemently opposed to stimulants being used so widely. He calls them "nuclear bombs," which should only be given under extreme circumstances and under close medical supervision. No one who talks about an “epidemic of overdiagnosis” denies that ADHD is a serious disorder that requires medication as well, but according to skeptics, the real numbers of people with ADHD have not changed and are about 5 percent, the rest they prescribe medicines undeservedly. nine0003
Take for life
Pharmacists are accused of aggressive marketing, which has led to the fact that poor academic performance or absent-mindedness already allows a child to be diagnosed and prescribed pills. Both parents and children themselves are convinced that stimulants will help to achieve success. Shire paid for 50,000 copies of a comic a few years ago in which superheroes encourage kids to take medicine to help them learn. Maroon 5 musician Adam Levine took part in Shire's advertising campaign aimed at teenagers. Pamphlets were printed for parents explaining that the pills would solve all the behavioral problems of their children. To remove stigma and encourage people to seek medical attention, lists of "Famous People with ADHD" have been prepared. Thus parents saw that their children were in good company with Thomas Edison, Abraham Lincoln, Galileo and Socrates. nine0003
Both parents and children themselves are convinced that stimulants will help to achieve success. Shire paid for 50,000 copies of a comic a few years ago in which superheroes encourage kids to take medicine to help them learn. Maroon 5 musician Adam Levine took part in Shire's advertising campaign aimed at teenagers. Pamphlets were printed for parents explaining that the pills would solve all the behavioral problems of their children. To remove stigma and encourage people to seek medical attention, lists of "Famous People with ADHD" have been prepared. Thus parents saw that their children were in good company with Thomas Edison, Abraham Lincoln, Galileo and Socrates. nine0003
Related Content:
Since 2000, the Food and Drug Administration (FDA) has repeatedly urged pharmaceutical companies to withdraw advertisements that exaggerate the effects of drugs. Although studies confirm that serious problems can occur in adulthood without ADHD treatment, stimulants as a therapy have not been proven to have all the benefits reported in advertisements, comics and booklets. In February 2017, Shire was ordered to pay $57.5 million in fines from the FDA for improperly advertising a range of drugs, including Vyvanse, Adderall XR, and Daytrana. nine0003
In February 2017, Shire was ordered to pay $57.5 million in fines from the FDA for improperly advertising a range of drugs, including Vyvanse, Adderall XR, and Daytrana. nine0003
ABC host Ty Pennington was also reprimanded for advertising stimulants. In his show, he talked about the amazing success that adults can achieve with stimulants, and admitted that Adderall changed his own life. A psychiatrist who was invited on the air developed the theme, adding that the majority of prisoners are people with undiagnosed ADHD who should be prescribed stimulants. At the same time, Pennington kept silent that he was a representative of Shire from 2006 to 2008 and received money in the company for this. Because he clearly exaggerated the positive effects of Adderall and kept silent about the negative side effects, the FDA issued him a warning. “I am not a medical expert. I'm a TV presenter," Pennington commented on his blunder. nine0003
Image: Ownyouradhd.com
Doctors, on whom diagnoses and prescriptions depend, were processed no less actively. For example, a new modification of Adderall - Adderall XR - was presented by Shire in April 2002 at the Ritz-Carlton Hotel and Spa in Pasadena, California. 70 doctors were invited to the event, who were told that ADHD without treatment threatens with lack of work or underemployment, fatal car accidents, criminal activities, unwanted pregnancies and sexually transmitted diseases. It did not mention that the studies did not evaluate whether stimulants were able to reduce these risks. Psychiatrist William Dodson, who hosted this presentation, told colleagues that he diagnoses about 300 patients a year and always recommends taking stimulants for the rest of his life. It was reported that for this performance, he received $2,000 from the Shire, and in 2010-2011, pharmaceutical companies transferred him $45,500. However, he himself believes that he is working as an educator, and statements that pharmacists force doctors to make incorrect diagnoses in order to increase sales are nothing more than conspiracy theories.
For example, a new modification of Adderall - Adderall XR - was presented by Shire in April 2002 at the Ritz-Carlton Hotel and Spa in Pasadena, California. 70 doctors were invited to the event, who were told that ADHD without treatment threatens with lack of work or underemployment, fatal car accidents, criminal activities, unwanted pregnancies and sexually transmitted diseases. It did not mention that the studies did not evaluate whether stimulants were able to reduce these risks. Psychiatrist William Dodson, who hosted this presentation, told colleagues that he diagnoses about 300 patients a year and always recommends taking stimulants for the rest of his life. It was reported that for this performance, he received $2,000 from the Shire, and in 2010-2011, pharmaceutical companies transferred him $45,500. However, he himself believes that he is working as an educator, and statements that pharmacists force doctors to make incorrect diagnoses in order to increase sales are nothing more than conspiracy theories. “If people need help, my job is to make sure they get it,” Dr. Dodson said. nine0003
“If people need help, my job is to make sure they get it,” Dr. Dodson said. nine0003
Legal doping
Stimulants have long crossed the threshold of medical offices. Many people do not have ADHD but are willing to take Adderall and other medications for performance or relaxation purposes (they also have a euphoric effect). Thanks to the advertising campaign, such drugs have developed a much more respectable image than dirty street stimulants. Unsurprisingly, up to 35.6 percent of American students are estimated to take Adderall to boost their cognitive abilities and prepare for last-minute exams. Twitter mentions of the drug have been noted to spike in December and April, when university exams are days away. nine0003
Related materials:
Athletes also love stimulants. For example, amphetamines have been taken, almost openly, by professional baseball players for decades. In 2005, Major League Baseball announced the start of a decisive fight against stimulants and the introduction of additional testing. Since then, a number of professional baseball players have reported that they suffer from ADHD. Immediately after the introduction of additional doping tests, the number of people receiving prescription stimulants increased from 28 players to 103, which amounted to 8 percent of all players in the league. In 2013 there were already 119. Dr. Gary Wadler of the World Anti-Doping Agency ironically remarked that there was an epidemic of ADHD in the Major Leagues. Such doping is no less popular in eSports. In 2015, the Electronic Sports League (ESL Gaming) added Adderall to its list of substances banned from tournament play and began testing players for drugs. The ban came after a well-known professional player admitted in an interview that his entire team used Adderall during an ESL tournament.
Since then, a number of professional baseball players have reported that they suffer from ADHD. Immediately after the introduction of additional doping tests, the number of people receiving prescription stimulants increased from 28 players to 103, which amounted to 8 percent of all players in the league. In 2013 there were already 119. Dr. Gary Wadler of the World Anti-Doping Agency ironically remarked that there was an epidemic of ADHD in the Major Leagues. Such doping is no less popular in eSports. In 2015, the Electronic Sports League (ESL Gaming) added Adderall to its list of substances banned from tournament play and began testing players for drugs. The ban came after a well-known professional player admitted in an interview that his entire team used Adderall during an ESL tournament.
According to experts, it is completely impossible to tell which proportion of adults who have been prescribed Adderall or other similar drugs actually have ADHD, and which are receiving the drug illegally. But there are obviously many such cases. As lovers of pharmacy stimulants admit, if you want to get a prescription, it is not difficult, which in the end can turn into a fairly strong addiction. In 2013, the Partnership for Drug-Free Children released the results of a survey in which nine percent of adolescents (about 1.9million) reported abuse of stimulants prescribed for ADHD. The Citizens Commission on Human Rights (CCHR) has stated that the criteria for diagnosing ADHD are so subjective and arbitrary that children and teens can easily feign to get a prescription, which they no doubt use.
But there are obviously many such cases. As lovers of pharmacy stimulants admit, if you want to get a prescription, it is not difficult, which in the end can turn into a fairly strong addiction. In 2013, the Partnership for Drug-Free Children released the results of a survey in which nine percent of adolescents (about 1.9million) reported abuse of stimulants prescribed for ADHD. The Citizens Commission on Human Rights (CCHR) has stated that the criteria for diagnosing ADHD are so subjective and arbitrary that children and teens can easily feign to get a prescription, which they no doubt use.
Richard Fee
Photo: The Richard Scott Fee Foundation
While the abuse of prescription stimulants is on the rise, even staunch opponents can't deny that in some cases they can actually improve cognitive performance and concentration significantly. Side effects include sleep disturbance, high blood pressure, heart problems, in rare cases, hallucinations and suicidal thoughts. nine0003
nine0003
Related materials:
If in the case of the "opioid epidemic" provoked by OxyContin, the number of deaths is tens of thousands per year, tragic cases associated with the abuse of stimulants are rare, although quite impressive. A seven-year-old child is known to have become depressed after taking medication, and a case has been reported in which the child began to experience severe hallucinations due to stimulants. 24-year-old Richard Fe experienced serious mental problems while trying to overcome his addiction to pharmaceutical stimulants, and ended up committing suicide. nine0003
Tragic events like this are perceived as collateral damage and are unlikely to cause the industry to change its mind. Pharmaceutical companies are investing millions in research and promotion of their products, so in the near future the market will only grow, and the number of people who cannot imagine their lives without stimulants will continue to increase.
Bupropion for attention deficit hyperactivity disorder (ADHD) in adults
Review question
We reviewed the evidence regarding the effects of bupropion in adults with ADHD. We also looked at adverse effects (side effects resulting from taking this medication).
We also looked at adverse effects (side effects resulting from taking this medication).
Relevance
ADHD is a brain disorder characterized by inattention, impulsivity and/or overactivity that interferes with normal functioning or development. Some people with ADHD have problems with only one aspect of their behavior, while others have problems in combination. Bupropion is a medication used to treat depression and quit smoking, but it is also used to treat ADHD. People with ADHD are commonly prescribed stimulant medications such as methylphenidate and amphetamines. However, not everyone responds well to these medications. Some people cannot tolerate stimulants due to side effects. Others may have medical reasons for taking stimulants, such as psychiatric or tic disorders. Other people may not want to use stimulants because they are controlled substances. Therefore, non-stimulant agents such as bupropion are sometimes used instead. Its efficacy in the treatment of ADHD remains unclear. nine0003
nine0003
Search date
Evidence is current to February 2017.
Study profile
We included six randomized controlled trials (RCTs), studies in which participants are randomly assigned to one of two or more treatment groups. Five studies were conducted in the US and the sixth in Iran. The studies involved 438 people with ADHD. All studies evaluated long-acting bupropion, a slow-absorbing formulation that can be taken as little as once a day. This simple dosage is suitable for people with ADHD, as the illness can cause them to forget to take their medications. nine0003
The duration of the studies varied from six to 10 weeks. The participants were all diagnosed with ADHD and often had other mental health issues. In one study, all participants had ADHD and were dependent on opioids (pain relievers).
Research funding sources
Four studies were funded by industry and the other two by public funds. In one of the publicly funded studies, the lead author was paid by the industry (not by the buproprion manufacturers) for research activities.