Wellbutrin chest pain
Acute myocardial infarction following bupropion (Zyban) | QJM: An International Journal of Medicine
Navbar Search Filter QJM: An International Journal of MedicineThis issueMedicine and HealthBooksJournalsOxford Academic Mobile Enter search term
Close
Navbar Search Filter QJM: An International Journal of MedicineThis issueMedicine and HealthBooksJournalsOxford Academic Enter search term
Advanced Search
Journal Article
R.N. Patterson,
R.N. Patterson
Search for other works by this author on:
Oxford Academic
PubMed
Google Scholar
N. A. Herity
N.A. Herity
Search for other works by this author on:
Oxford Academic
PubMed
Google Scholar
QJM: An International Journal of Medicine, Volume 95, Issue 1, January 2002, Pages 58–59, https://doi.org/10.1093/qjmed/95.1.58
Published:
01 January 2002
Navbar Search Filter QJM: An International Journal of MedicineThis issueMedicine and HealthBooksJournalsOxford Academic Mobile Enter search term
Close
Navbar Search Filter QJM: An International Journal of MedicineThis issueMedicine and HealthBooksJournalsOxford Academic Enter search term
Advanced Search
Sir,
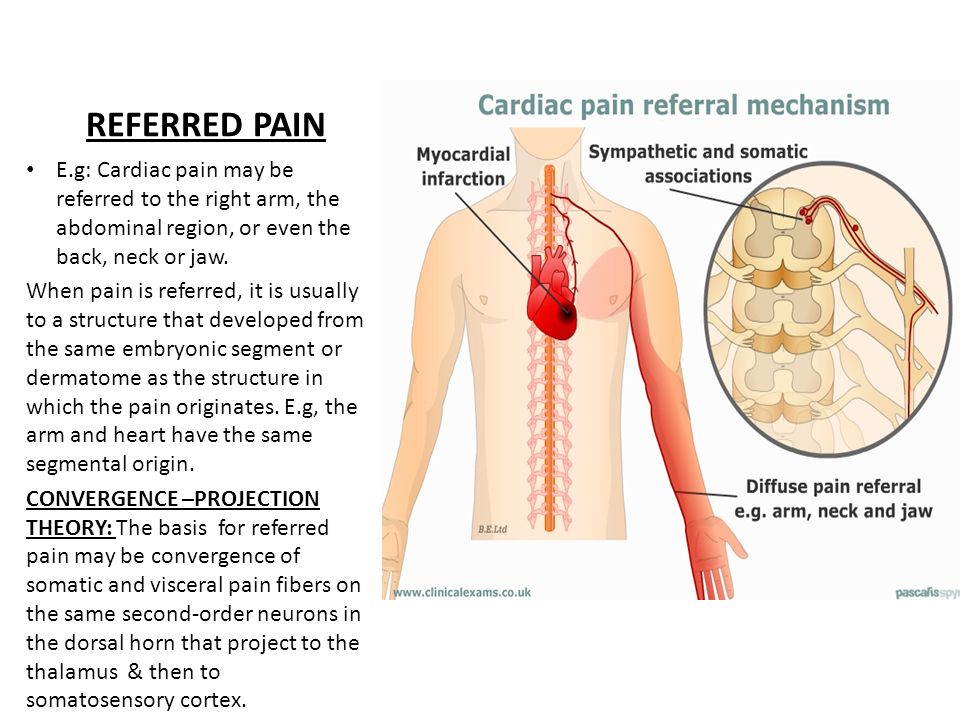
Bupropion (Zyban) was licensed by the Medicines Control Agency in the UK in June 2000 as an aid to smoking cessation. Subsequent reports of 18 deaths raised questions about its safety.1 We describe a patient who presented with acute myocardial infarction, whose symptoms of chest pain can be dated to commencing bupropion 2 weeks previously.
Subsequent reports of 18 deaths raised questions about its safety.1 We describe a patient who presented with acute myocardial infarction, whose symptoms of chest pain can be dated to commencing bupropion 2 weeks previously.
A 43‐year‐old insurance clerk presented with classical symptoms of acute inferoposterolateral myocardial infarction. Thrombolytic therapy was administered with resolution of ST segments. Troponin I was >500 iU/l, and he completed 12 min of the modified Bruce protocol and was discharged on secondary prevention therapy. Risk factors included a positive family history and cigarette smoking of 26 pack‐years. He had no past medical history of note, was on no regular medication and took little regular exercise. Two weeks previously, he had commenced bupropion 150 mg in an attempt to stop smoking. Prior to this he had not experienced chest pain. The dose was increased as per the suggested dosing regimen, and 7 days later he stopped smoking. At 10 days, he developed episodes of central chest and arm pain and the following day discontinued bupropion. Three days later he presented with acute myocardial infarction.
Three days later he presented with acute myocardial infarction.
Bupropion has been licensed as an adjunct to smoking cessation in the USA since 1998. Originally prescribed as an antidepressant, its antismoking properties are believed to be based on central dopaminergic properties and noradrenergic activity. The UK launch has stimulated public and media interest. Public concern has been heightened by reports of severe adverse reactions including seizures and deaths. Despite this high profile, the scientific literature contains only one prospective safety study in the treatment of depression2 and few detailed adverse event reports. US Public Health Service guidelines3 suggest that additional research into the relative efficacy and safety of this group of drugs is required. National guidelines4 make little reference to safety concerns.
Up to 30 April 2001, there were 238 reports of chest pain and 134 reports of chest tightness to the Committee on the Safety of Medicines among an estimated 390 000 patients receiving bupropion in the UK.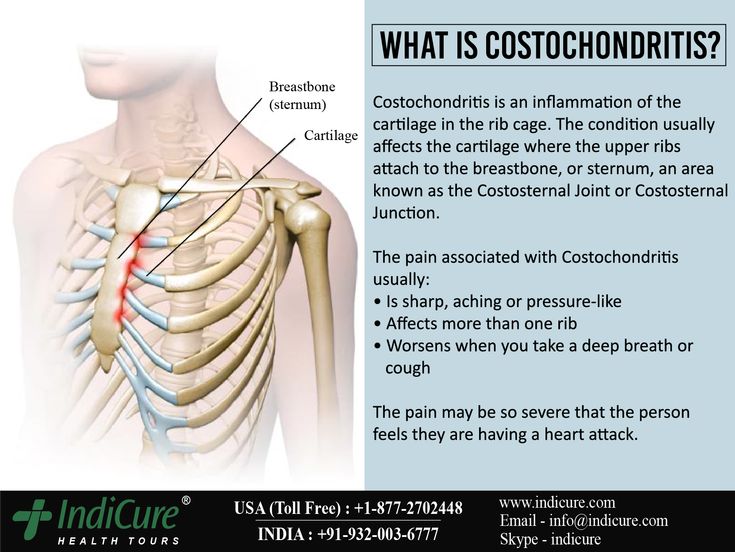 Thirty‐seven deaths have been attributed to underlying comorbidity. While associations between bupropion, fatalities and myocardial infarction may be coincidental, only by vigilance, comprehensive reporting and investigation of all such cases will causal relationships be detected. Severe coronary artery spasm has previously been described with bupropion and pseudoephedrine,5 supporting a plausible link with myocardial infarction.
Thirty‐seven deaths have been attributed to underlying comorbidity. While associations between bupropion, fatalities and myocardial infarction may be coincidental, only by vigilance, comprehensive reporting and investigation of all such cases will causal relationships be detected. Severe coronary artery spasm has previously been described with bupropion and pseudoephedrine,5 supporting a plausible link with myocardial infarction.
The freedom to smoke comes at a price to society. While all attempts to reduce smoking rates are to be supported, maintaining public confidence in such therapies requires comprehensive and prospective demonstration of safety. As with nicotine replacement therapy, the public perception of safety is at least as important as its actual efficacy in ensuring that smoking cessation therapy is a public health success.
1
BBC News Online 18 February 2001; http://news.bbc.co.uk/hi/english/health/newsid_1177000/1177137. stm
stm
2
Dunner DL, Zisook S, Billow AA, et al. A prospective safety surveillance study for bupropion sustained‐release in the treatment of depression.
J Clin Psychiat
1998
;
59
:
366
–73.
3
West R, McNeill A, Raw M. Smoking cessation guidelines for health professionals: An update.
Thorax
2000
;
55
:
987
–99.
4
US Public Health Service.
Treating tobacco use and dependence
. Rockville, MD: Agency for Healthcare Research Quality, June 2000. http://hstat.nlm.nih.gov/ftrs/pick?collect=ahcpr&dbName=tob&cd=1&t=997462747
5
Pederson KJ, Kuntz DH, Garbe GJ.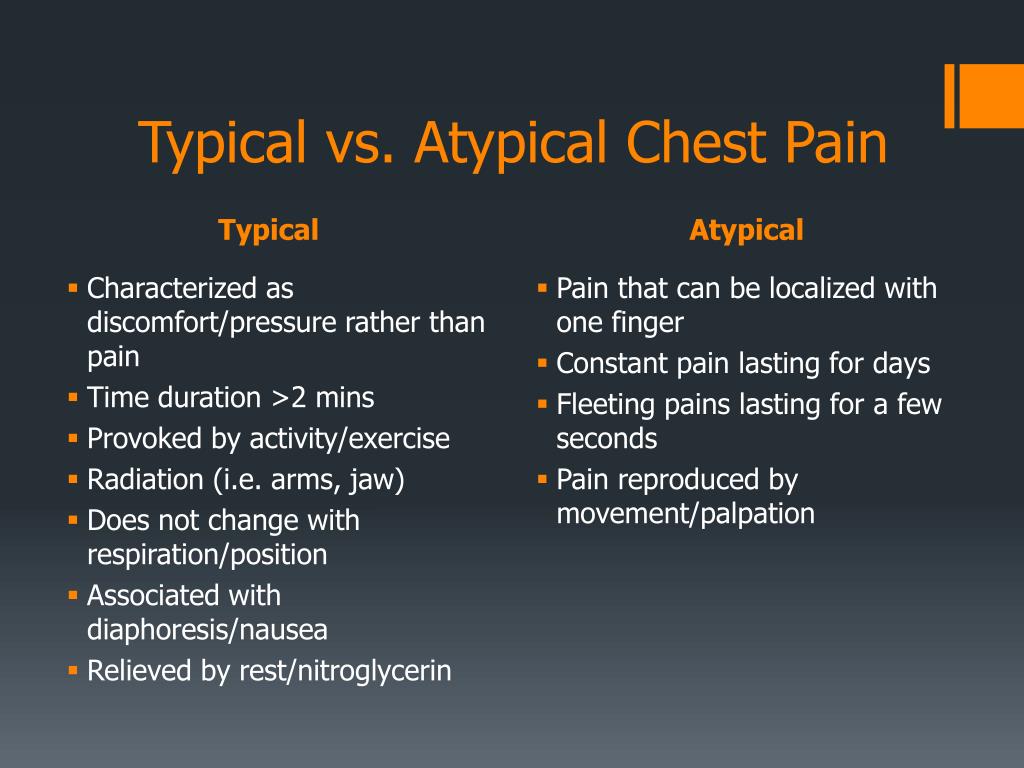 Acute myocardial ischaemia associated with ingestion of bupropion and pseudoephedrine in a 21‐year‐old man.
Acute myocardial ischaemia associated with ingestion of bupropion and pseudoephedrine in a 21‐year‐old man.
Can J Cardiol
2001
;
17
:
599
–601.
© Association of Physicians
Issue Section:
Correspondence
Download all slides
Advertisement intended for healthcare professionals
Citations
Altmetric
More metrics information
Email alerts
Article activity alert
Advance article alerts
New issue alert
Receive exclusive offers and updates from Oxford Academic
Citing articles via
-
Latest
-
Most Read
-
Most Cited
Thyroid Hemiagenesis
Angioimmunoblastic T-cell lymphoma: an immunological masquerade
Leprosy & Broken Bacilli on Slit Skin Smear
See Saw Nystagmus Associated with Retinitis Pigmentosa
Cortico-bulbar excitability in abductor laryngeal dystonia disease: the diagnostic role of transcranial magnetic stimulation
Advertisement intended for healthcare professionals
Common and Rare Side Effects for Wellbutrin XL
COMMON side effects
If experienced, these tend to have a Severe expression i
If experienced, these tend to have a Less Severe expression i
INFREQUENT side effects
If experienced, these tend to have a Severe expression i
If experienced, these tend to have a Less Severe expression i
RARE side effects
If experienced, these tend to have a Severe expression i
If experienced, these tend to have a Less Severe expression i
Full Drug Information
Free RX Coupon
Save up to 80% on your prescriptions.
Available coupons
Save up to 80% on your prescription with WebMDRx
Related Links
Drug Survey
Are you currently using Wellbutrin XL?
This survey is being conducted by the WebMD marketing sciences department.
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.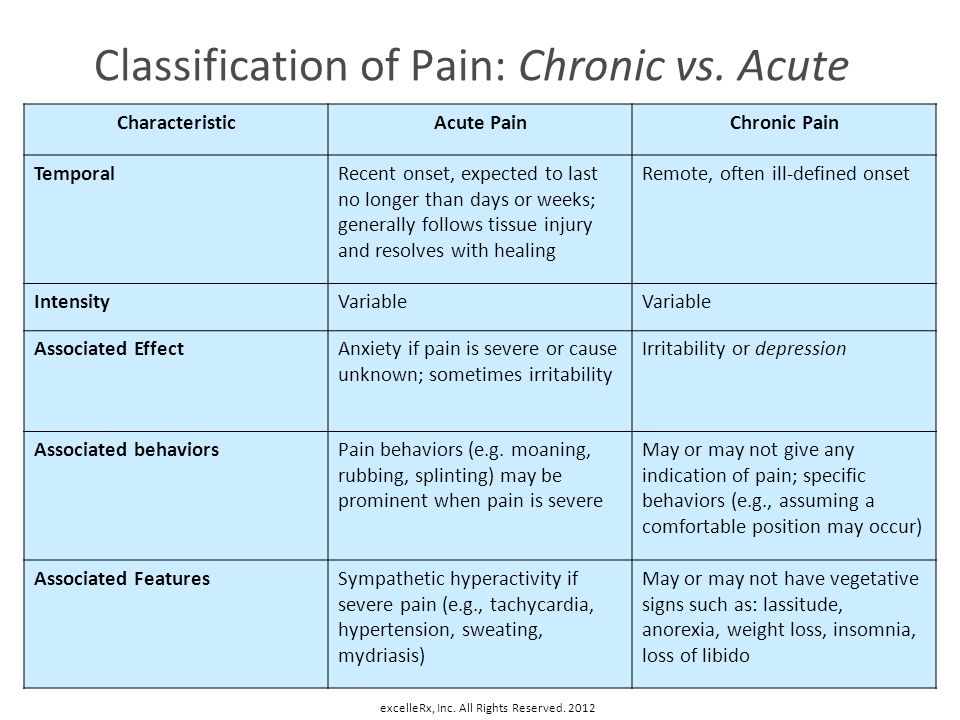
Memorial Sloan Kettering Cancer Center
Adult Medication
ShareProvided by Lexicomp ® , this document contains all the information you need to know about this medicine, including indications, directions for use, side effects, and when your healthcare provider should be contacted.
Trade names: USA
Aplenzin; Forfivo XL; Wellbutrin SR; Wellbutrin XL; Zyban[DSC]
Trade names: Canada
MYLAN-BuPROPion XL [DSC]; ODAN Bupropion SR; TARO-Bupropion XL; TEVA-Bupropion XL; Wellbutrin SR; Wellbutrin XL; Zyban
Warning
- Drugs like this have increased the likelihood of suicidal thoughts or actions in children and young people. This risk may be higher in people who have tried or had suicidal thoughts in the past. All people taking this drug must be closely monitored. If you develop or worsen disorders such as depression, nervousness, anxiety, grouchiness, panic attacks, and changes in mood or behavior, contact your doctor immediately.
 Contact your doctor immediately if you have suicidal thoughts or suicide attempts.
Contact your doctor immediately if you have suicidal thoughts or suicide attempts.
What is this drug used for?
- Used to treat depression.
- Used to prevent seasonal affective disorder (SAD).
- Used to stop smoking.
- This drug may also be used for other indications. Consult your doctor.
What should I tell my doctor BEFORE taking this drug?
- If you have an allergy to this drug, any of its ingredients, other drugs, foods or substances. Tell your doctor about your allergies and how they have manifested.
- If you have ever had seizures.
- If you abuse alcohol and suddenly stopped using it.
- If you are taking certain other drugs, such as anticonvulsants or tranquilizers, and you stop taking them abruptly.
- If you have ever had an eating disorder such as anorexia or bulimia.
- If you have any of the following health conditions: kidney disease or liver disease.
- If you have taken medications for depression or Parkinson's disease in the past 14 days. These include isocarboxazid, phenelzine, tranylcypromine, selegiline, or rasagiline. An episode of very high blood pressure may occur.
- If you are taking any of the following drugs: linezolid or methylene blue.
- If you are taking another drug that contains the same medicine.
This list of drugs and conditions that may interact with this drug is not exhaustive.
Tell your doctor and pharmacist about all medicines you take (both prescription and over-the-counter, natural products and vitamins) and any health problems you have. You need to make sure that this drug is safe for your conditions and in combination with other drugs you are already taking. Do not start or stop taking any drug or change the dosage without your doctor's advice.
What do I need to know or do while taking this drug?
For all patients taking this drug:
- Tell all your health care workers that you are taking this drug.
 These are doctors, nurses, pharmacists and dentists.
These are doctors, nurses, pharmacists and dentists. - Avoid driving or doing other tasks or jobs that require alertness or keen eyesight until you know how this drug affects you.
- This drug may affect the results of some lab tests. Tell all your health care workers and laboratory staff that you are taking this drug.
- Do not stop taking this drug abruptly without consulting your doctor. This can increase the risk of side effects. If necessary, taking this drug should be stopped gradually, in accordance with the recommendations of the doctor.
- High blood pressure has happened with this drug. Monitor your blood pressure as directed by your doctor.
- This drug may increase the risk of seizures. The risk may be increased in people who take higher doses of the drug, who have certain health problems, or who use certain other drugs. People who abruptly stop drinking large amounts of alcohol or abruptly stop taking certain medications (such as drugs used for anxiety, insomnia, or seizures) are also at higher risk.
 Talk to your doctor to find out if you have an increased risk of seizures.
Talk to your doctor to find out if you have an increased risk of seizures. - Avoid drinking alcohol while taking this drug.
- Check with your doctor before using marijuana, other forms of cannabis, or prescription or over-the-counter drugs that can slow you down.
- It may take several weeks to achieve full effect.
- This drug is not approved for use in children. Consult your doctor.
- If you are 65 years of age or older, use this drug with caution. You may experience more side effects.
- Tell your doctor if you are pregnant, planning to become pregnant, or breastfeeding. The benefits and risks for you and your child will need to be discussed.
If you smoke:
- Not all drugs are approved for use to help you quit smoking. Check with your doctor to make sure you get the right drug.
- When using bupropion for smoking cessation, the appearance of new or aggravation of existing disorders of the psyche, mood or behavior was noted.
 These disturbances include suicidal or homicidal thoughts, depression, violent acts, rage, anxiety and anger. These disorders have been observed in people with and without mental and mood disorders in the past. Consult your doctor.
These disturbances include suicidal or homicidal thoughts, depression, violent acts, rage, anxiety and anger. These disorders have been observed in people with and without mental and mood disorders in the past. Consult your doctor.
What side effects should I report to my doctor immediately?
WARNING. In rare cases, this drug can cause serious and sometimes deadly side effects in some patients. Contact your doctor or seek medical attention right away if you have any of the following signs or symptoms that may be associated with serious side effects:
- Signs of an allergic reaction, such as rash, hives, itching, red and swollen skin with blisters or peeling, possibly accompanied by fever, wheezing or wheezing, tightness in the chest or throat, difficulty breathing, swallowing or speaking, unusual hoarseness, swelling in the mouth, face, lips, tongue or throat.
- Signs of high blood pressure, such as a very severe headache, or dizziness, or loss of consciousness, or blurred vision.
- Feelings of confusion, inability to concentrate, or changes in behavior.
- Hallucinations (a person sees or hears something that is not in reality).
- If, after starting the medication, seizures become more frequent or severe.
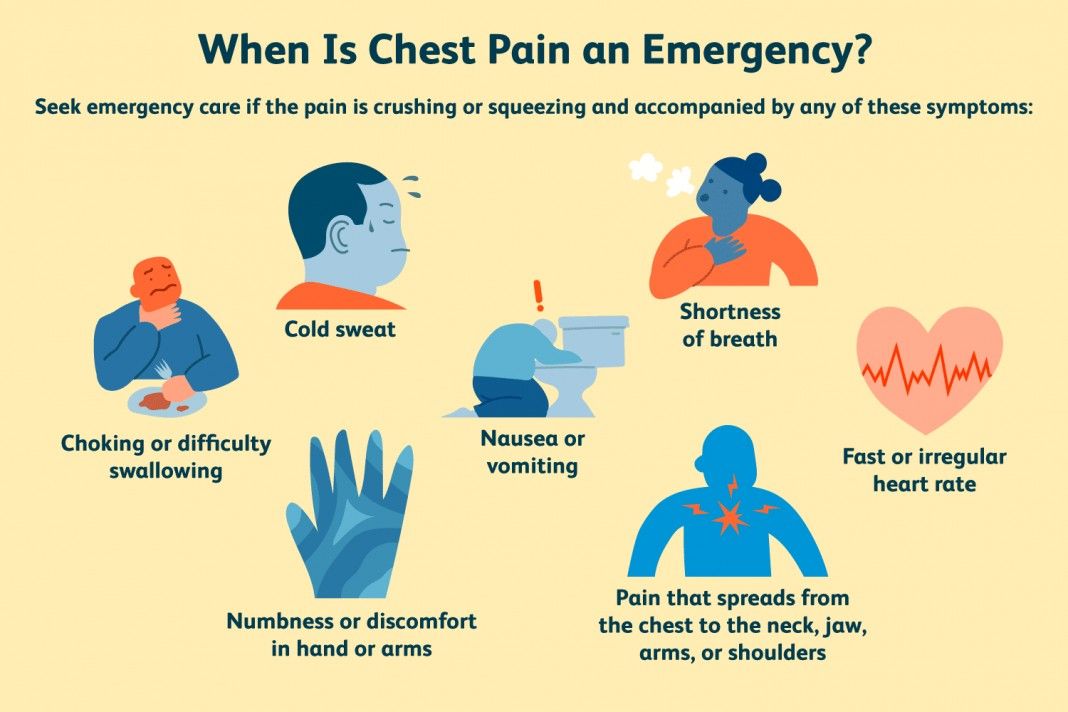
- Chest pain, angina pectoris, tachycardia, or abnormal heart rhythm.
- Inflammation.
- Dyspnea.
- Hearing change.
- Tinnitus.
- Frequent urination.
- Swelling of the gland.
- Violation of motor functions.
- Some patients may be at increased risk of eye problems when using this drug. Your doctor may order an eye examination to see if you are at increased risk for these eye problems. Call your doctor right away if you have eye pain, vision changes, swelling or redness around the eye.
- Possible severe skin reaction (Stevens-Johnson syndrome/toxic epidermal necrolysis). This can lead to severe health problems, which can be permanent, and sometimes death.
 Seek immediate medical attention if you experience symptoms such as redness, swelling of the skin with blistering or peeling (with or without fever), redness or irritation of the eyes, and sores in the mouth, throat, nose, or eyes.
Seek immediate medical attention if you experience symptoms such as redness, swelling of the skin with blistering or peeling (with or without fever), redness or irritation of the eyes, and sores in the mouth, throat, nose, or eyes.
What are some other side effects of this drug?
Any medicine can have side effects. However, for many people, side effects are either minor or non-existent. Contact your doctor or seek medical attention if these or any other side effects bother you or do not go away:
All formulations:
- Dizziness or headache.
- Constipation, diarrhea, abdominal pain, nausea, vomiting, or decreased appetite.
- Shiver.
- Nervous tension and agitation.
- Strange or unusual dreams.
- Gas formation.
- Dry mouth.
- Sleep disorders.
- Pain in the joints or muscles.
- Irritation of the nose or throat.
- Excessive sweating.
- Unexplained fluctuations in weight.
Extended release tablets:
- The tablet shell of some brand name drugs can sometimes be seen in the stool. For drugs of these brands, this is normal and does not cause concern. If you have any questions, please consult your doctor.
This list of possible side effects is not exhaustive. If you have any questions about side effects, please contact your doctor. Talk to your doctor about side effects.
You can report side effects to the National Health Board.
You can report side effects to the FDA at 1-800-332-1088. You can also report side effects at https://www.fda.gov/medwatch.
What is the best way to take this drug?
Use this drug as directed by your doctor. Read all the information provided to you. Strictly follow all instructions.
For all uses of this drug:
- Do not take the drug more often than prescribed. This may increase your risk of developing seizures. Make sure you know at what intervals you need to use the drug.

- Take in the morning if you are taking this drug once a day.
- Take this drug with or without food.
- If you cannot sleep, do not take this drug at bedtime. Consult your doctor.
- Swallow whole. Do not chew, break or crush.
- Keep taking this drug as instructed by your doctor or other health care professional, even if you feel well.
- If you have difficulty swallowing, check with your doctor.
For smoking cessation:
- You can take this drug for 1 week before you stop smoking.
- Nicotine replacement and psychological help can be done at the same time for best results.
- If you have not been able to stop smoking after 12 weeks of taking this drug, talk with your doctor.
- When you try to quit smoking, you may experience nicotine withdrawal symptoms even if you use quit smoking drugs like this drug. There are many signs of nicotine withdrawal. People who are trying to quit smoking have occasionally experienced depression and suicidal thoughts.
 Consult with your physician.
Consult with your physician.
What if I miss a dose of a drug?
- Skip the forgotten dose and return to your regular schedule.
- Do not take 2 doses or an additional dose at the same time.
How do I store and/or discard this drug?
- Store at room temperature, protected from light. Store in a dry place. Do not store in the bathroom.
- Keep all medicines in a safe place. Keep all medicines out of the reach of children and pets.
- Dispose of unused or expired drugs. Do not empty into a toilet or sewer unless instructed to do so. If you have any questions about disposing of medicines, ask your pharmacist. Drug disposal programs may be in place in your area.
General information about medicines
- If your health does not improve or even worsens, see your doctor.
- Do not give your medicine to anyone and do not take other people's medicines.
- Some medicines may come with other patient information leaflets.
 If you have questions about this drug, talk with your doctor, nurse, pharmacist, or other health care professional.
If you have questions about this drug, talk with your doctor, nurse, pharmacist, or other health care professional. - A separate instruction for patients is attached to the drug. Please read this information carefully. Reread it each time you refill your supply. If you have any questions about this drug, ask your doctor, pharmacist, or other health care professional.
- If you think you have overdosed, call a poison control center or get medical help right away. Be prepared to tell or show what drug you took, how much, and when it happened.
Consumer Use of Information and Limitation of Liability
This summary information includes a summary of the diagnosis, treatment, and/or drug product. It is not intended to be a comprehensive source of data and should be used as a tool to help the user understand and/or evaluate potential diagnostic and treatment options. It does NOT include all information about conditions, treatments, medications, side effects, or risks that may apply to a particular patient. It should not be considered medical advice or a substitute for medical advice, diagnosis or treatment provided by a physician based on a medical examination and assessment of the patient's specific and unique circumstances. Patients should consult with their physician for full information about their health, medical issues, and treatment options, including any risks or benefits regarding the use of medications. This information is not a guarantee that a treatment or drug is safe, effective, or approved for a particular patient. UpToDate, Inc. and its subsidiaries disclaim any warranties or liabilities related to this information or its use. The use of this information is subject to the Terms of Use found at https://www.wolterskluwer.com/en/know/clinical-effectiveness-terms.
It should not be considered medical advice or a substitute for medical advice, diagnosis or treatment provided by a physician based on a medical examination and assessment of the patient's specific and unique circumstances. Patients should consult with their physician for full information about their health, medical issues, and treatment options, including any risks or benefits regarding the use of medications. This information is not a guarantee that a treatment or drug is safe, effective, or approved for a particular patient. UpToDate, Inc. and its subsidiaries disclaim any warranties or liabilities related to this information or its use. The use of this information is subject to the Terms of Use found at https://www.wolterskluwer.com/en/know/clinical-effectiveness-terms.
Last revision date
2021-07-28
Copyright
© UpToDate, Inc. and its affiliates and/or licensors, 2023. All rights reserved.
Date last updated
Monday, December 12, 2022
instruction, use, analogues of the drug, composition, indications, contraindications, side effects in the reference book of medicines from UNIAN
Application of Wellbutrin
Wellbutrin - composition and form of release of the drug
Wellbutrin: how to take the drug
Wellbutrin - contraindications, side effects
Analogues of Wellbutrin
Wellbutrin - psychoanaleptic, antidepressant.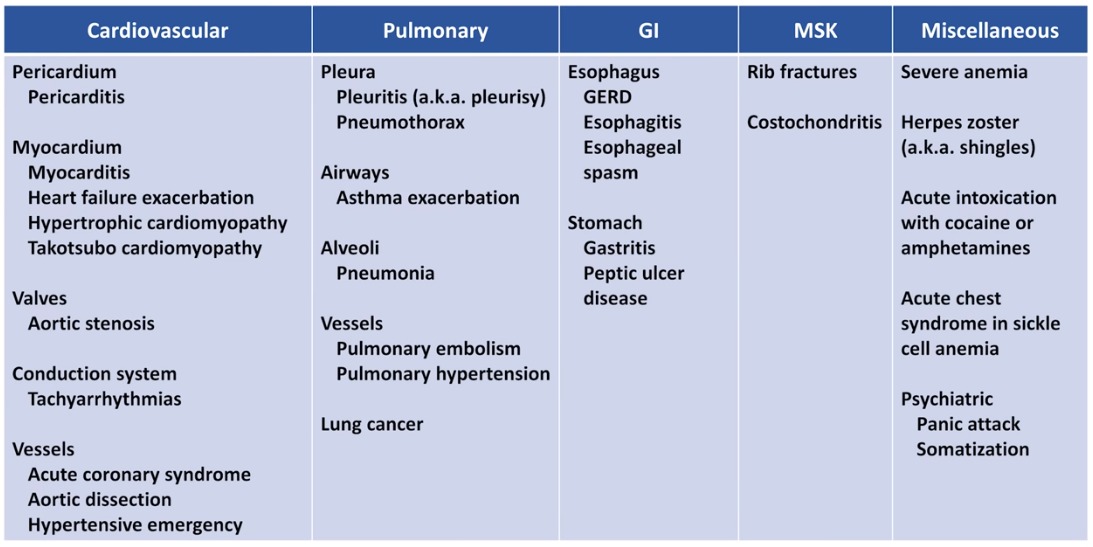
Application of Wellbutrin
Indication.
Treatment of major depressive conditions.
Wellbutrin - composition and form of release of the drug
excipients: microcrystalline cellulose, hypromellose, cysteine hydrochloride monohydrate, magnesium stearate, white dye concentrate (Opadry OY-7300 White or Opadry YS-1-18202-A White), carnauba wax, black food coloring.
Dosage form. Coated tablets, prolonged release.
Wellbutrin: how to take the drug
Dosage and administration.
The drug begins to act no earlier than 14 days after the start of therapy. As with other antidepressants, the full effect of the drug is observed only after a few weeks of treatment.
Wellbutrin tablets should be swallowed whole and not divided, crushed or chewed as this may increase the risk of side effects, including convulsions.
The maximum single dose should not exceed 150 mg. Use Wellbutrin tablets in 2 doses per day with an interval between doses of at least 8 hours.
Wellbutrin - contraindications, side effects
Contraindications.
Wellbutrin is contraindicated in patients with hypersensitivity to bupropion or to any of the components of the drug.
Wellbutrin is contraindicated in patients with seizures.
Wellbutrin is contraindicated in patients who are currently abruptly ceasing alcohol or sedatives.
Wellbutrin tablets contain bupropion and therefore should not be given to patients receiving any other drug containing bupropion as the incidence of seizures is dose dependent.
Wellbutrin is contraindicated in patients with current or history of bulimia nervosa or anorexia nervosa, because in this group of patients there was a greater incidence of seizures when prescribing the rapid release form of bupropion.
Co-administration of Wellbutrin and monoamine oxidase inhibitors is contraindicated. Between the abolition of irreversible MAO inhibitors and the start of treatment with Wellbutrin, at least 14 days should elapse.
Adverse reactions.
From the immune system: hypersensitivity reactions such as urticaria, more severe hypersensitivity reactions including angioedema, dyspnea/bronchospasm or anaphylactic shock.
From the side of metabolism and digestive disorders: anorexia, weight loss, impaired blood glucose levels.
On the part of the psyche: insomnia, sleep disturbance, agitation, anxiety, depression, dysphoria, disorientation, aggressiveness, hostility, irritability, anxiety, hallucinations, unusual dreams, depersonalization, delusions, paranoid thinking, suicidal thoughts and suicidal behavior, psychosis , euphoria, mania, hypomania, changes in mental state.
From the side of the central nervous system: headache, tremor, dizziness, taste disturbances, memory disturbances, anxiety, myoclonus, dystonia, migraine, attention disorders, vertigo, akathisia, dysarthria, convulsions, EEG disturbances, dystonia, ataxia, parkinsonism, motor coordination disorder, memory impairment, paresthesia, syncope, coma, delirium, sensory disturbances, dyskinesia.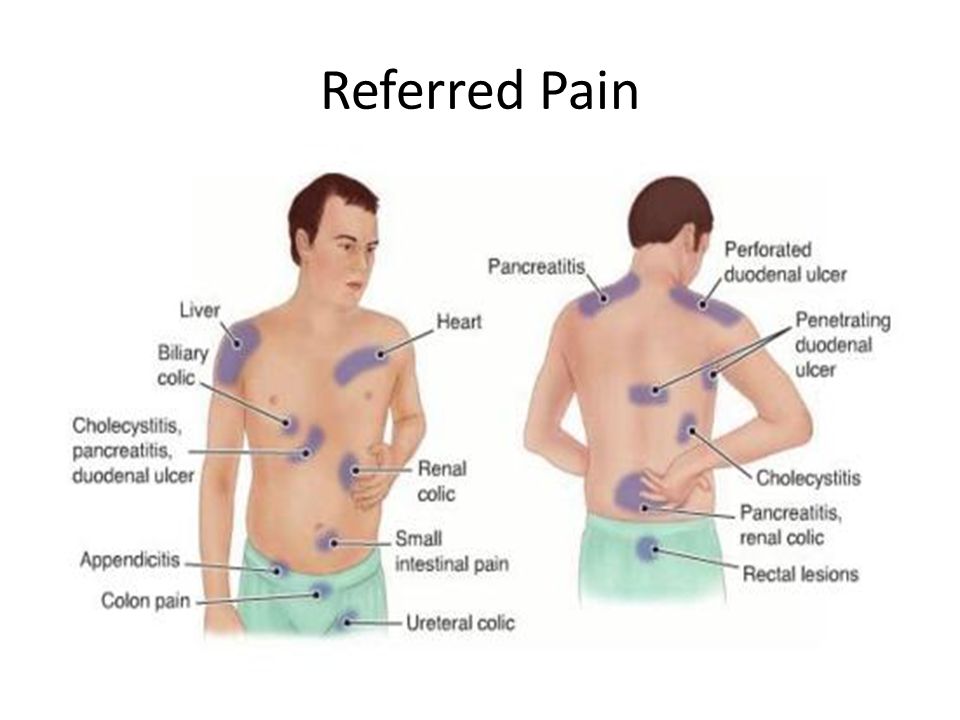
On the part of the organs of vision: visual disturbance, diplopia, mydriasis, increased eye pressure.
From the hearing organs: ringing in the ears.
From the side of the heart: tachycardia, cardiac arrhythmias, ECG changes, myocardial infarction, increased blood pressure, edema, palpitations.
Vascular: increased blood pressure (in some cases significant), redness, vasodilation, postural hypotension.
From the digestive tract: dry mouth, gastrointestinal disorders, including nausea and vomiting, abdominal pain, constipation, dyspepsia, toothache, gum irritation, intestinal perforation.
From the side of the hepatobiliary system: increased levels of liver enzymes, jaundice, hepatitis.
From the side of the skin and subcutaneous tissue: rash, itching, sweating, polymorphic erythema and Stevens-Johnson syndrome, exacerbation of psoriasis, alopecia.
From the respiratory system: pulmonary embolism, bronchitis.
 N. Patterson, N.A. Herity, Acute myocardial infarction following bupropion (Zyban), QJM: An International Journal of Medicine, Volume 95, Issue 1, January 2002, Pages 58–59, https://doi.org/10.1093/qjmed/95.1.58
N. Patterson, N.A. Herity, Acute myocardial infarction following bupropion (Zyban), QJM: An International Journal of Medicine, Volume 95, Issue 1, January 2002, Pages 58–59, https://doi.org/10.1093/qjmed/95.1.58