Side effects of abilify shot
Abilify Maintena intramuscular: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing
Warnings:
There may be a slightly increased risk of serious, possibly fatal side effects (such as stroke, heart failure, fast/irregular heartbeat, pneumonia) when this medication is used by older adults with dementia. This medication is not approved for the treatment of dementia-related behavior problems. Discuss the risks and benefits of this medication, as well as other effective and possibly safer treatments for dementia-related behavior problems, with the doctor.
How to use Abilify Maintena Suspension,Extended Release Vial
Read the Medication Guide provided by your pharmacist before you start using aripiprazole and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
The extended-release injection should only be used if you have already taken aripiprazole by mouth without any serious side effects.
Aripiprazole is given by injection into the buttock or upper arm muscle by a health care professional, usually once every month. Some doses may be given once every 6 weeks or once every 2 months. Do not rub/massage the injection site after your dose.
After your first injection, your doctor may direct you to continue to take your antipsychotic medication by mouth (such as aripiprazole tablet/solution) for 2 to 3 weeks. This will help maintain the right level of medication in your body as you switch from receiving medication by mouth to receiving it by injection. Follow your doctor's instructions carefully.
The dosage is based on your medical condition, response to treatment, and other medications you may be taking. Be sure to tell your doctor and pharmacist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
It may take several weeks to get the full benefit of this medication. Use this medication regularly to get the most benefit from it. To help you remember, mark the days on the calendar when you need to receive the medication.
To help you remember, mark the days on the calendar when you need to receive the medication.
Tell your doctor if your condition does not improve or if it worsens.
Side Effects
See also Warning section.
Dizziness, lightheadedness, drowsiness, tiredness, blurred vision, weight gain, shaking (tremors), and redness/pain/swelling at the injection site may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.
Dizziness and lightheadedness can increase the risk of falling. Get up slowly when rising from a sitting or lying position.
Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
Tell your doctor right away if you have any serious side effects, including: fainting, trouble swallowing, restlessness (especially in the legs), seizures, trouble controlling certain urges (such as gambling, sex, eating or shopping), interrupted breathing during sleep.
This medication may rarely make your blood sugar rise, which can cause or worsen diabetes. Tell your doctor right away if you have symptoms of high blood sugar such as increased thirst/urination. If you already have diabetes, check your blood sugar regularly as directed and share the results with your doctor. Your doctor may need to adjust your diabetes medication, exercise program, or diet.
This medication may rarely cause a condition known as tardive dyskinesia. In some cases, this condition may be permanent. Tell your doctor right away if you develop any unusual uncontrolled movements (especially of the face, mouth, tongue, arms, or legs).
This medication may rarely cause a serious condition called neuroleptic malignant syndrome (NMS). Get medical help right away if you develop any of the following: fever, muscle cramps/stiffness/pain/tenderness/weakness, severe tiredness, severe confusion, sweating, fast/irregular heartbeat, dark urine, signs of kidney problems (such as change in the amount of urine).
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: fever, swollen lymph nodes, rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US - Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
Precautions
See also Warning and Side Effects sections.
Before using aripiprazole, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: problems with receiving injections into the buttocks, problems with blood flow in the brain (such as cerebrovascular disease, stroke), blood clotting problems (such as hemophilia, low platelets), diabetes (including family history), heart problems (such as low blood pressure, coronary artery disease), nervous system problems (such as dementia, NMS, seizures), obesity, low white blood cell count (including history of low white blood cell count caused by medications), swallowing problems, breathing trouble during sleep (sleep apnea).
This drug may make you dizzy or drowsy or blur your vision. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. Do not drive, use machinery, or do anything that needs alertness or clear vision until you can do it safely. Avoid alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).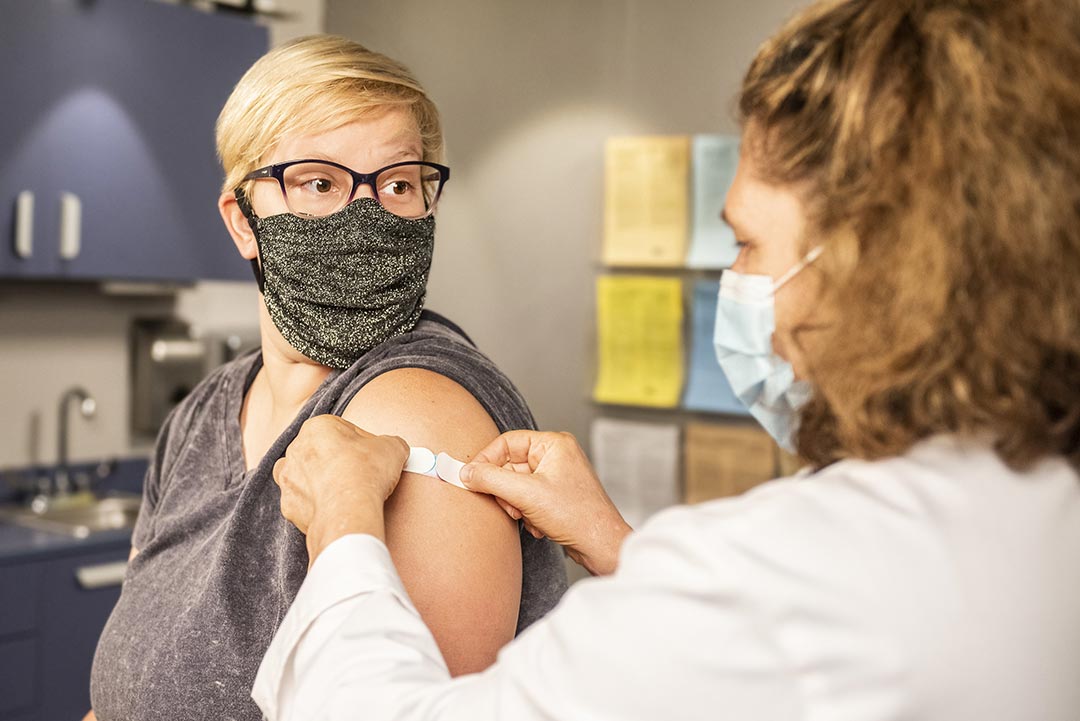
This medication may make you sweat less, making you more likely to get heat stroke. Avoid doing things that may cause you to overheat, such as hard work or exercise in hot weather, or using hot tubs. When the weather is hot, drink a lot of fluids and dress lightly. If you overheat, quickly look for a place to cool down and rest. Get medical help right away if you have a fever that does not go away, mental/mood changes, headache, or dizziness.
Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
Older adults may be more sensitive to the side effects of this drug, especially seizures, drowsiness, dizziness, lightheadedness, confusion, tardive dyskinesia, swallowing problems, and other serious (rarely fatal) side effects. (See also Warning section.) Drowsiness, dizziness, lightheadedness, and confusion can increase the risk of falling.
During pregnancy, this medication should be used only when clearly needed. Babies born to mothers who have used this drug during the last 3 months of pregnancy may rarely develop symptoms including muscle stiffness or shakiness, drowsiness, feeding/breathing difficulties, or constant crying. If you notice any of these symptoms in your newborn especially during their first month, tell the doctor right away.
Babies born to mothers who have used this drug during the last 3 months of pregnancy may rarely develop symptoms including muscle stiffness or shakiness, drowsiness, feeding/breathing difficulties, or constant crying. If you notice any of these symptoms in your newborn especially during their first month, tell the doctor right away.
Since untreated mental/mood problems (such as schizophrenia) can be a serious condition, do not stop taking this medication unless directed by your doctor. If you are planning pregnancy, become pregnant, or think you may be pregnant, immediately discuss with your doctor the benefits and risks of using this medication during pregnancy.
This medication passes into breast milk. Consult your doctor before breast-feeding.
Consult your pharmacist or physician.
Interactions
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
A product that may interact with this drug is: metoclopramide.
Tell your doctor or pharmacist if you are taking other products that cause drowsiness such as opioid pain or cough relievers (such as codeine, hydrocodone), alcohol, marijuana (cannabis), drugs for sleep or anxiety (such as alprazolam, lorazepam, zolpidem), muscle relaxants (such as carisoprodol, cyclobenzaprine), or antihistamines (such as cetirizine, diphenhydramine).
Check the labels on all your medicines (such as allergy or cough-and-cold products) because they may contain ingredients that cause drowsiness. Ask your pharmacist about using those products safely.
Does Abilify Maintena Suspension,Extended Release Vial interact with other drugs you are taking?
Enter your medication into the WebMD interaction checker
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include: very fast heartbeat, loss of consciousness.
Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include: very fast heartbeat, loss of consciousness.
Laboratory and/or medical tests (such as blood sugar, weight, cholesterol/triglyceride levels) may be performed from time to time to monitor your progress or check for side effects. Consult your doctor for more details.
It is important to get each dose of this medication as scheduled. If you miss a dose, ask your doctor or pharmacist right away for a new dosing schedule. Your doctor may direct you to also take aripiprazole by mouth again for some time if more than a certain number of weeks have passed since your last injection. Follow your doctor's instructions carefully.
Not applicable. This medication is given in a hospital or clinic and will not be stored at home.
Look up another drug
Find other drugs that treat your condition
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.
Side Effects of ABILIFY MAINTENA® (aripiprazole)
Side Effects of ABILIFY MAINTENA® (aripiprazole) Skip to main contentIt’s important to understand the benefits and risks when you and your healthcare provider are considering treatment.
 The more you know about them, the better prepared you may be to talk with your healthcare provider about them. In a clinical trial, the most common side effects of ABILIFY MAINTENA were:
The more you know about them, the better prepared you may be to talk with your healthcare provider about them. In a clinical trial, the most common side effects of ABILIFY MAINTENA were:Weight gain
Inner sense of restlessness (feeling like you need to move)
Injection site pain
Sleepiness (sedation)
These are not all the possible side effects of ABILIFY MAINTENA.
Please read IMPORTANT SAFETY INFORMATION below.
Get information to start the conversation with your healthcare provider about ABILIFY MAINTENAStart Now
IMPORTANT SAFETY INFORMATION
Elderly people with dementia-related psychosis are at increased risk of death when treated with antipsychotic medicines including ABILIFY MAINTENA. ABILIFY MAINTENA is not for the treatment of people who have lost touch with reality (psychosis) due to confusion and memory loss (dementia).
Do not receive ABILIFY MAINTENA if you are allergic to aripiprazole or any of the ingredients in ABILIFY MAINTENA. Allergic reactions to aripiprazole have ranged from rash, hives and itching to anaphylaxis, which may include difficulty breathing, tightness in the chest, and swelling of the mouth, face, lips, or tongue.
Allergic reactions to aripiprazole have ranged from rash, hives and itching to anaphylaxis, which may include difficulty breathing, tightness in the chest, and swelling of the mouth, face, lips, or tongue.
ABILIFY MAINTENA may cause serious side effects including:
-
feel very thirsty
-
need to urinate more than usual
-
feel very hungry
-
feel weak or tired
-
feel sick to your stomach
-
feel confused, or your breath smells fruity
- Increased fat levels (cholesterol and triglycerides) in your blood.
- Weight gain. You and your healthcare provider should check your weight regularly.
Do not drive, operate machinery, or do other dangerous activities until you know how ABILIFY MAINTENA affects you. ABILIFY MAINTENA may make you feel drowsy.
ABILIFY MAINTENA may make you feel drowsy.
Do not drink alcohol while you receive ABILIFY MAINTENA.
Before receiving ABILIFY MAINTENA, tell your healthcare provider about all your medical conditions, including if you:
-
have never taken aripiprazole before
-
have diabetes or high blood sugar or a family history of diabetes or high blood sugar. Your healthcare provider should check your blood sugar before you start receiving ABILIFY MAINTENA and during your treatment.
-
have or had seizures (convulsions)
-
have or had low or high blood pressure
-
have or had heart problems or a stroke
-
have or had a low white blood cell count
-
have problems that may affect you receiving an injection in your arm or buttocks
-
are pregnant or plan to become pregnant.
 It is not known if ABILIFY MAINTENA will harm your unborn baby. If you become pregnant while receiving ABILIFY MAINTENA, talk to your healthcare provider about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or go to http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/
It is not known if ABILIFY MAINTENA will harm your unborn baby. If you become pregnant while receiving ABILIFY MAINTENA, talk to your healthcare provider about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or go to http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/ -
are breastfeeding or plan to breastfeed. ABILIFY MAINTENA can pass into your milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you receive ABILIFY MAINTENA.
Tell your healthcare provider about all the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements.
ABILIFY MAINTENA and other medicines may affect each other causing possible serious side effects. Do not start or stop any medicines during treatment with ABILIFY MAINTENA without talking to your healthcare provider first.
The most common side effects of ABILIFY MAINTENA include: weight gain, inner sense of restlessness such as feeling like you need to move (akathisia), injection site pain, or sleepiness (sedation).
It is important to contact your healthcare provider if you experience prolonged, abnormal muscle spasms or contractions, which may be signs of a condition called dystonia.
These are not all the possible side effects of ABILIFY MAINTENA.
If you have any questions about your health or medicines, talk to your healthcare provider.
You are encouraged to report side effects of ABILIFY MAINTENA (aripiprazole). Please contact Otsuka America Pharmaceutical, Inc. at 1-800-438-9927 or FDA at 1-800-FDA-1088 (www.fda.gov/medwatch).
INDICATIONS
ABILIFY MAINTENA® (aripiprazole) is a prescription medicine given by injection by a healthcare professional for:
- treatment of schizophrenia in adults
- maintenance treatment of bipolar I disorder in adults
It is not known if ABILIFY MAINTENA is safe and effective in children under 18 years of age.
Please read , including BOXED WARNING, and .
IMPORTANT SAFETY INFORMATION
Elderly people with dementia-related psychosis are at increased risk of death when treated with antipsychotic medicines including ABILIFY MAINTENA. ABILIFY MAINTENA is not for the treatment of people who have lost touch with reality (psychosis) due to confusion and memory loss (dementia).
Do not receive ABILIFY MAINTENA if you are allergic to aripiprazole or any of the ingredients in ABILIFY MAINTENA. Allergic reactions to aripiprazole have ranged from rash, hives and itching to anaphylaxis, which may include difficulty breathing, tightness in the chest, and swelling of the mouth, face, lips, or tongue.
ABILIFY MAINTENA may cause serious side effects including:
-
feel very thirsty
-
need to urinate more than usual
-
feel very hungry
-
feel weak or tired
-
feel sick to your stomach
-
feel confused, or your breath smells fruity
- Increased fat levels (cholesterol and triglycerides) in your blood.
- Weight gain. You and your healthcare provider should check your weight regularly.
Do not drive, operate machinery, or do other dangerous activities until you know how ABILIFY MAINTENA affects you. ABILIFY MAINTENA may make you feel drowsy.
Do not drink alcohol while you receive ABILIFY MAINTENA.
Before receiving ABILIFY MAINTENA, tell your healthcare provider about all your medical conditions, including if you:
-
have never taken aripiprazole before
-
have diabetes or high blood sugar or a family history of diabetes or high blood sugar. Your healthcare provider should check your blood sugar before you start receiving ABILIFY MAINTENA and during your treatment.
-
have or had seizures (convulsions)
-
have or had low or high blood pressure
-
have or had heart problems or a stroke
-
have or had a low white blood cell count
-
have problems that may affect you receiving an injection in your arm or buttocks
-
are pregnant or plan to become pregnant.
It is not known if ABILIFY MAINTENA will harm your unborn baby. If you become pregnant while receiving ABILIFY MAINTENA, talk to your healthcare provider about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or go to http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/
-
are breastfeeding or plan to breastfeed. ABILIFY MAINTENA can pass into your milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you receive ABILIFY MAINTENA.
Tell your healthcare provider about all the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements.
ABILIFY MAINTENA and other medicines may affect each other causing possible serious side effects. Do not start or stop any medicines during treatment with ABILIFY MAINTENA without talking to your healthcare provider first.
The most common side effects of ABILIFY MAINTENA include: weight gain, inner sense of restlessness such as feeling like you need to move (akathisia), injection site pain, or sleepiness (sedation).
It is important to contact your healthcare provider if you experience prolonged, abnormal muscle spasms or contractions, which may be signs of a condition called dystonia.
These are not all the possible side effects of ABILIFY MAINTENA.
If you have any questions about your health or medicines, talk to your healthcare provider.
You are encouraged to report side effects of ABILIFY MAINTENA (aripiprazole). Please contact Otsuka America Pharmaceutical, Inc. at 1-800-438-9927 or FDA at 1-800-FDA-1088 (www.fda.gov/medwatch).
INDICATIONS
ABILIFY MAINTENA® (aripiprazole) is a prescription medicine given by injection by a healthcare professional for:
- treatment of schizophrenia in adults
- maintenance treatment of bipolar I disorder in adults
It is not known if ABILIFY MAINTENA is safe and effective in children under 18 years of age.
Please read , including BOXED WARNING, and .
↑
Abilify injection / Abilify Maintena Injection In Russian - Product
Abilify injection / Abilify Maintena Injection in Russian - Medicine.net 9000 .net
- Overview
- Benefits
- Side effects
- Precautions
- Interactions
- Contraindications
Overview
Abilify Injection / Abilify Maintena Injection is used for Schizophrenia, Major depressive disorder, Bipolar disorder, Irritability, Autism-related, Tourette's disorder and other conditions.
Abilify Injection / Abilify Maintena Injection contains Aripiprazole as an active ingredient. Available in injection form.
Detailed information regarding the use, composition, dosage, side effects of Abilify Injection / Abilify Maintena Injection, as well as user reviews are provided below:
Benefits
Abilify injection / Abilify Maintena Injection is used to treat, control, prevent and improve the following diseases, conditions and symptoms:
- Schizophrenia
- Bipolar Bipolar Disgree
- Annial
- Relyticism, related to Authetis, related to Authetis, related to Autheticism, related to Autheticism, related to auto Tourette
Learn more: Benefits of
Side Effects of
The following is a list of possible side effects that may occur from all constituents of Abilify Injection / Abilify Maintena Injection. This list is not final. These side effects have been recorded previously, but are not always recorded when using the drug. Some of these side effects may be extremely rare, but have incredibly severe consequences. If you notice any side effects, contact your doctor immediately. Especially in the case of observing side effects for a long time.
This list is not final. These side effects have been recorded previously, but are not always recorded when using the drug. Some of these side effects may be extremely rare, but have incredibly severe consequences. If you notice any side effects, contact your doctor immediately. Especially in the case of observing side effects for a long time.
- Nausea
- Rivot
- Card
- Pain 9000 dizziness
- Acatisia
- Late discinery
- Insomnia 9000
- Blood abnormalities
- Seizure
- Dysphagia
- Suicidal ideation
If you experience side effects not listed above, contact your healthcare provider for advice. In addition, you can report side effects to your local Food and Drug Administration.
Precautions
Before starting this drug, tell your doctor about any medications you are taking, dietary supplements (such as vitamins, natural supplements, etc. ), allergies, existing medical conditions, and current health conditions (such as pregnancy, upcoming surgery, and etc.). The side effects of the drug may be more pronounced depending on the state of your body. Take this medicine as directed by your doctor, or follow the directions for use that come with your medicine. The dosage of the drug depends on your condition. Tell your doctor if there is no change or if your condition worsens. Important points to discuss with your healthcare provider are listed below.
), allergies, existing medical conditions, and current health conditions (such as pregnancy, upcoming surgery, and etc.). The side effects of the drug may be more pronounced depending on the state of your body. Take this medicine as directed by your doctor, or follow the directions for use that come with your medicine. The dosage of the drug depends on your condition. Tell your doctor if there is no change or if your condition worsens. Important points to discuss with your healthcare provider are listed below.
- Alzheimer's disease
- Diabetes
- Do not stop taking this drug without medical advice
- Stay hydrated
- Susceptibility to hypotension
- Seizures
- or have diabetes
- Family history of diabetes
- Cardiovascular disease
- Use caution while driving or operating machinery until you know how this medicine will affect you
If you use other drugs or supplements at the same time as this drug, the effects of Abilify Injection / Abilify Maintena Injection may change. Tell your healthcare provider about all medications, vitamins, and supplements you use. Your doctor will be able to make the right plan for taking the drug, which will avoid negative interactions. Abilify Injection / Abilify Maintena Injection may interact with the following drugs and products:
Tell your healthcare provider about all medications, vitamins, and supplements you use. Your doctor will be able to make the right plan for taking the drug, which will avoid negative interactions. Abilify Injection / Abilify Maintena Injection may interact with the following drugs and products:
Is this drug (product) addictive or addictive?
Most drugs are not habit-forming or addictive. In most cases, the state classifies drugs that can be addictive as controlled dispensing drugs. For example, schedule H or X in India and schedule II-V in the USA. Please check the information on the drug packaging to make sure that this drug is not in the controlled category. Also, do not self-medicate or accustom your body to medications without consulting your doctor.
Can I stop using this product immediately or do I need to slowly stop using it?
Some medications need to be stopped gradually due to a rebound effect. Be sure to consult your healthcare provider for advice based on your body, general health, and other medications you may be taking.
Be sure to consult your healthcare provider for advice based on your body, general health, and other medications you may be taking.
Cite this page
Page URL
HTML Link
Abilify Injection
APA Style Citation
- Abilify Injection (n.d.). Retrieved October 05, 2022, from https://www.Medication.net/uk-ru/abilify-maintena-injection
MLA Style Citation
- Abilify Injection " Tabletwise.com . N.p., n.d. Web. 05 Oct. 2022.
Chicago Style Citation
- "Abilify Injection / Abilify Maintena Injection in English - Product - Medicine.net" Tabletwise. Accessed October 05, 2022. https://www.Medication.net/uk-ru/abilify-maintena-injection.
More information about Abilify Injection / Abilify Maintena Injection
- Uses of
- Reviews
- What are the uses of Abilify Injection / Abilify Maintena Injection?
- What are the side effects of Abilify Injection / Abilify Maintena Injection?
- What other medicines does Abilify Injection / Abilify Maintena Injection interact with?
- When should you not take Abilify Injection / Abilify Maintena Injection?
- What precautions should you take while using Abilify Injection / Abilify Maintena Injection?
Last updated date
This page was updated on 9/27/2020.
This page provides information for Abilify Maintena Injection Product in English .
Business
Share with friends, get 20% off
Invite your friends to TabletWise learning marketplace. For each purchase they make, you get 20% off (upto $10) on your next purchase.
Smart pills as an ethical issue - expert material, Lahta Clinic
On November 13, 2017, the US Food and Drug Administration (FDA) issued a decision that immediately became the focus of attention of the world medical community and became the subject of the most wide discussion. The FDA, the most authoritative organization, whose opinion is heeded all over the world, "only" approved the drug Abilify MySite for use in clinical practice. The seemingly ordinary procedure for certification of a medicinal product gave rise to heated discussions and again raised a wave of public interest in such a painful at all times, urgent and acute problem as medical ethics.
The terms “smart pill” (mostly dietary supplements) and “smart case for pills”, as well as several pharmaceutical trademarks registered in the post-Soviet territory using the word “smart”, have nothing to do with that “smart pill "(eng. "smart pill"), which is devoted to this review.
"smart pill"), which is devoted to this review.
Let's start with the trade name "Abilify MyCite". It is very difficult to translate this game of words-neologisms into Russian: something like “Give me the capacity ...” or “Give back the possibilities in my case (precedent)”. Previously, the drug "Abilify Maintena" (approx. translation "Source of Opportunities Supporting") has already been approved for use, certified in the Russian Federation under the current registration number LS-001812 dated 11/16/09and sold in pharmacies under the brand name Abilify.
The drug was developed and manufactured by one of the largest Japanese pharmaceutical corporations - Otsuka Pharmaceutical Co., Ltd. In America, Abilify is distributed jointly with Bristol-Myers Squibb (New York), a company in whose name, by the way, a Russian registration certificate has been issued. However, it was Otsuka who submitted the recently approved application for certification to the FDA. This time, she is performing in a somewhat unusual collaboration for a pharmaceutical giant with Proteus Digital Health, a Silicon Valley digital technology developer.
Abilify is the trade name for aripiprazole, one of the newer atypical neuroleptics. In turn, antipsychotics are a group of antipsychotic drugs that, since the second half of the 1990s, have become one of the breakthroughs in psychopharmacology, having a number of undeniable advantages over the "traditional" or "typical" first antipsychotics - chlorpromazine and haloperidol. Like other drugs in this group, aripiprazole is used primarily in the treatment of endogenous "major psychoses" - schizophrenia, bipolar disorder and psychotic depression; clinical trials are also underway for the efficacy of aripiprazole in the treatment of alcoholism. This drug has already proven itself quite well in psychiatric practice and, according to the results of comparative studies, surpasses clozapine, olanzapine, risperidone, etc., released a little earlier, in a number of indicators. The FDA approved the use of aripiprazole in the United States in 2002.
And today, after 15 years, Abilify with a new clarifying name is again in the spotlight. For the first time, a mass-produced dosage form containing the so-called. oral or "swallowed" tracer - a microscopic sealed radio frequency emitter that is located inside a standard capsule with the active substance. Once in the intestine, the emitter is activated and sends a signal to a miniature sensor-transmitter, which is attached, like a regular adhesive plaster, to the patient's stomach. This transmitter, in turn, transmits information about the medication to the smartphone. Thus, the patient can keep track of the time of admission and the number of doses he has already taken; in addition, he can provide the doctor and caregivers with access to this information through a dedicated web portal.
For the first time, a mass-produced dosage form containing the so-called. oral or "swallowed" tracer - a microscopic sealed radio frequency emitter that is located inside a standard capsule with the active substance. Once in the intestine, the emitter is activated and sends a signal to a miniature sensor-transmitter, which is attached, like a regular adhesive plaster, to the patient's stomach. This transmitter, in turn, transmits information about the medication to the smartphone. Thus, the patient can keep track of the time of admission and the number of doses he has already taken; in addition, he can provide the doctor and caregivers with access to this information through a dedicated web portal.
Mitchell Mathis, director of psychiatric drugs at the FDA's Center for Drug Research and Evaluation, believes that the ability to monitor prescription medications can be beneficial for some patients with mental disorders. According to M. Mathis - which, apparently, should be considered a reflection of the official position, since they are quoted in a press release - “FDA supports the development and application of new technology, therefore it intends to work with companies in studying all its benefits for patients and doctors. "
"
So Abilify as such was certified 15 years ago; the oral sensor used in the new dosage form received FDA approval back in 2012. However, the official press release says nothing about the fact that a year and a half ago, an application for certification of a hybrid drug-and-device form was already submitted by Otsuka - and was rejected. The FDA then required companies to provide additional information about the intended use of the product and associated human risk factors, as well as to confirm that the hybrid drug can be safely and effectively used by patients.
In response, Otsuka and Proteus expressed “disappointment with the regulator’s decision”, which did not surprise commentators: a year and a half ago, a lot was written about the fact that the rejection of the application causes serious financial damage to companies, given the funds spent on the upgraded Abilify and fierce competition in the market (formerly only from authorized generic manufacturers of this drug).
Today's discussions are dominated by restrained joy at the advancement of new technologies, but there is also some bewilderment. So, commentator Rebecca Robbins notes: forgetfulness and twilight consciousness can accompany those mental disorders for which Abilify is approved for treatment, and patients have to make a lot of effort to take the required medications on time. However, the new drug will come with a warning on the packaging: "Technology has not been proven to help patients take their medications as prescribed."
Immediately following the FDA decision, the journal Anesthesia & Analgesia published the results of a mini-study conducted in Boston on a sample of 15 patients who had suffered fractures and were prescribed opioid analgesics. The study used smart tablets similar to Abilify. The paper's lead author, Dr. Edward Boyer, says the new technology is highly promising, saying it provides a "direct measure" of how many opioids are being taken, which in some cases could be a warning sign of developing tolerance or dependence.
Professor of Psychiatry Tia P. Powell (Albert Einstein College of Medicine, New York) looks at the news from a completely different perspective.
“Unfortunately,” Dr. Powell's article begins, “unfortunately, the first FDA-approved smart pill was a drug used in the treatment of schizophrenia and bipolar disorder. This again raises confusing ethical questions."
Clinical experience and decades of research, it goes on to say, confirm that off-label drug use is a major problem in all branches of medicine. Smart pills can help forgetful people with diabetes, heart problems, and other drug-dependent patients who would like to achieve more and feel better with a prescribed treatment regimen. There is no doubt that, says Prof. Powell that mental illness drastically reduces the quality of life and its duration, and in this regard, drugs can really be useful. It is the WHO who defines mental disorders as the “major contributor” to the global damage from diseases in general. Psychotic episodes are typical of patients who are not taking psychiatrist-prescribed drugs, and such episodes not only further damage the brain, but often endanger both the patients themselves and others. Compliance with prescriptions for patients with mental illness is just as important as for somatic patients. What makes the high-tech version of aripiprazole problematic, however, is that this hybrid drug can easily be used in coercive treatment that ignores the values and preferences of the mentally ill themselves. In general, forced therapy is a long and painful problem in the history of psychiatry. In certain situations, a person with a mental disorder may be subjected, regardless of consent or disagreement, to inpatient or outpatient treatment, sometimes with compulsory medication. Of course, the best medicine in the world won't help, notes Tia P. Powell, if you don't take it. But only at 19In 1970, the US Supreme Court for the first time recognized a legal deficit in people hospitalized against their will.
Psychotic episodes are typical of patients who are not taking psychiatrist-prescribed drugs, and such episodes not only further damage the brain, but often endanger both the patients themselves and others. Compliance with prescriptions for patients with mental illness is just as important as for somatic patients. What makes the high-tech version of aripiprazole problematic, however, is that this hybrid drug can easily be used in coercive treatment that ignores the values and preferences of the mentally ill themselves. In general, forced therapy is a long and painful problem in the history of psychiatry. In certain situations, a person with a mental disorder may be subjected, regardless of consent or disagreement, to inpatient or outpatient treatment, sometimes with compulsory medication. Of course, the best medicine in the world won't help, notes Tia P. Powell, if you don't take it. But only at 19In 1970, the US Supreme Court for the first time recognized a legal deficit in people hospitalized against their will. Both clinicians and patients around the world struggle to strike the right balance between safety and individual rights.
Both clinicians and patients around the world struggle to strike the right balance between safety and individual rights.
A new dosage form is at the very center of this struggle. Manufacturers assure us that patients will consent to such treatment and the use of tracking technology. However, this smart tablet is very attractive for judicial applications. For those patients who are ordered to take drugs by the court, "consent" takes on a very different meaning if it is exchanged for freedom, custody of a child, or a reduced sentence.
I bet, says T.P. Powell, that people will look for ways to outsmart the tracker, while the manufacturers of this expensive drug have gone to great lengths to make it harder to disable the tracker. But I'd rather see an innovation that would serve to build trust and collaboration between doctor and patient.
We need to take a closer look not only at the benefits of Abilify MySite's hybrid drug, the article says, but also at the risks associated with it. It will help some people take their medication as prescribed, but it can also be a high-tech form of psychiatric coercion. If the new drug improves the treatment of "major psychoses", then it is necessary to carefully consider who, in fact, will benefit and who may suffer.
It will help some people take their medication as prescribed, but it can also be a high-tech form of psychiatric coercion. If the new drug improves the treatment of "major psychoses", then it is necessary to carefully consider who, in fact, will benefit and who may suffer.
Abilify MySite has been approved without any indication of its ethical use. “I am convinced,” Professor Powell concludes, “that both clinicians and consumers should be involved in the development of such ethical guidelines. True progress in psychiatry involves a genuine respect for those who struggle with mental illness, and not just the development of new ways to apply treatment to these people.
The publication caused hot and sometimes diametrically opposed comments from specialists, patients, their relatives and people who are simply not indifferent to the problem.
Robert P.N. Shirin: “Professor Powell seems to be inclined to think of the new drug as something like a Trojan horse in the mouth. I wonder if she sees an ethical problem in DOT (the so-called "Treatment under direct supervision" - approx. Lakhta Clinic), which is a common practice in the fight against tuberculosis?
Steven J. Leitner: "The article ignores the fact that, say, long-acting drugs have been around in psychiatry for decades and perform essentially the same function."
Marie Lutz: “There is absolutely no evidence that psychotic episodes “destroy the brain”… Another question, and it is not raised in the article, is who and how will justify compulsory treatment with an antipsychotic that has such extensive and serious side effects. effects such as weight gain, tardive dyskinesia, akathisia and reduced life expectancy. In addition, it is doubtful that if atypical antipsychotics can help some people (usually for a short time), then they should necessarily be taken by all other patients. Personally, I believe that the new drug is a "bad faith development" and should be recalled.
Karen D'Angelo: “While I wholeheartedly support a patient's right to choose, as a neighbor of a schizophrenic patient, I also see the consequences of patients not taking their prescribed medications when the judicial system is overly concerned with ensuring compliance (compliance is therapeutic union of a doctor and a patient - approx.