Side effects effexor 150 mg
Effexor XR Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing
Warnings:
Antidepressant medications are used to treat a variety of conditions, including depression and other mental/mood disorders. These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
Tell the doctor right away if you notice worsening depression/other psychiatric conditions, unusual behavior changes (including possible suicidal thoughts/attempts), or other mental/mood changes (including new/worsening anxiety, panic attacks, trouble sleeping, irritability, hostile/angry feelings, impulsive actions, severe restlessness, very rapid speech). Be especially watchful for these symptoms when a new antidepressant is started or when the dose is changed.
Warnings:
Antidepressant medications are used to treat a variety of conditions, including depression and other mental/mood disorders. These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
Tell the doctor right away if you notice worsening depression/other psychiatric conditions, unusual behavior changes (including possible suicidal thoughts/attempts), or other mental/mood changes (including new/worsening anxiety, panic attacks, trouble sleeping, irritability, hostile/angry feelings, impulsive actions, severe restlessness, very rapid speech). Be especially watchful for these symptoms when a new antidepressant is started or when the dose is changed.
Be especially watchful for these symptoms when a new antidepressant is started or when the dose is changed.
... Show More
Uses
Venlafaxine is used to treat depression, anxiety, panic attacks, and social anxiety disorder (social phobia). It may improve your mood and energy level and may help restore your interest in daily living. It may also decrease fear, anxiety, unwanted thoughts, and the number of panic attacks. Venlafaxine is known as a serotonin-norepinephrine reuptake inhibitor (SNRI). It works by helping to restore the balance of certain natural substances (serotonin and norepinephrine) in the brain.
How to use Effexor XR
Read the Medication Guide and, if available, the Patient Information Leaflet provided by your pharmacist before you start using venlafaxine and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
Take this medication by mouth as directed by your doctor, usually once daily with food, either in the morning or evening.
Do not crush, chew, or dissolve this medication. Doing so can release all of the drug at once, increasing the risk of side effects. Swallow whole without crushing or chewing.
If you are taking the capsules, swallow them whole. If you have trouble swallowing the capsules whole, you may open the capsule and sprinkle the contents onto a spoonful of applesauce. Swallow all of the mixture right away without chewing. Drink a glass of water after each dose.
The dosage is based on your medical condition and response to treatment. To reduce your risk of side effects, your doctor may direct you to start this medication at a low dose and gradually increase your dose. Follow your doctor's instructions carefully. Take this medication regularly to get the most benefit from it. To help you remember, take it at the same time each day.
Keep taking this medication even if you feel well. Do not stop taking this medication without consulting your doctor. Some conditions may become worse when this drug is suddenly stopped. Also, you may experience symptoms such as confusion, mood swings, blurred vision, headache, tiredness, sleep changes, and brief feelings similar to electric shock. Your dose may need to be gradually decreased to reduce side effects. Report any new or worsening symptoms right away.
Also, you may experience symptoms such as confusion, mood swings, blurred vision, headache, tiredness, sleep changes, and brief feelings similar to electric shock. Your dose may need to be gradually decreased to reduce side effects. Report any new or worsening symptoms right away.
It may take several weeks to feel the benefit of this medication. Tell your doctor if your condition lasts or gets worse.
Side Effects
See also Warning section.
Nausea, drowsiness, dizziness, dry mouth, constipation, loss of appetite, blurred vision, nervousness, trouble sleeping, unusual sweating, or yawning may occur. If any of these effects last or get worse, tell your doctor promptly.
Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
This medication may raise your blood pressure. Check your blood pressure regularly and tell your doctor if the results are high.
Tell your doctor right away if you have any serious side effects, including: easy bruising/bleeding, decreased interest in sex, changes in sexual ability, muscle cramps/weakness, shaking (tremor).
Get medical help right away if you have any very serious side effects, including: cough that doesn't go away, shortness of breath, chest pain, severe/pounding headache, black/bloody stools, vomit that looks like coffee grounds, eye pain/swelling/redness, widened pupils, vision changes (such as seeing rainbows around lights at night), seizure.
This medication may increase serotonin and rarely cause a very serious condition called serotonin syndrome/toxicity. The risk increases if you are also taking other drugs that increase serotonin, so tell your doctor or pharmacist of all the drugs you take (see Drug Interactions section). Get medical help right away if you develop some of the following symptoms: fast heartbeat, hallucinations, loss of coordination, severe dizziness, severe nausea/vomiting/diarrhea, twitching muscles, unexplained fever, unusual agitation/restlessness.
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US - Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
Precautions
Before taking venlafaxine, tell your doctor or pharmacist if you are allergic to it; or to desvenlafaxine; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: bleeding problems, personal or family history of glaucoma (angle-closure type), high blood pressure, heart problems (such as heart failure, previous heart attack), high cholesterol, kidney disease, liver disease, seizure disorder, thyroid disease.
This drug may make you dizzy or drowsy or blur your vision. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. Do not drive, use machinery, or do anything that needs alertness or clear vision until you can do it safely. Avoid alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).
Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
Older adults may be more sensitive to the side effects of this drug, especially dizziness when standing. Older adults may also be more likely to develop a type of salt imbalance (hyponatremia), especially if they are taking "water pills" (diuretics). Dizziness and salt imbalance can increase the risk of falling. Older adults may also be at greater risk for bleeding while using this drug.
Dizziness and salt imbalance can increase the risk of falling. Older adults may also be at greater risk for bleeding while using this drug.
Children may be more sensitive to the side effects of the drug, especially loss of appetite and weight loss. Monitor weight and height in children who are taking this drug.
During pregnancy, this medication should be used only when clearly needed. It may harm an unborn baby. Also, babies born to mothers who have used this drug during the last 3 months of pregnancy may rarely develop withdrawal symptoms such as feeding/breathing difficulties, seizures, muscle stiffness, or constant crying. If you notice any of these symptoms in your newborn, tell the doctor promptly.
Since untreated mental/mood problems (such as depression, anxiety, panic attacks) can be a serious condition, do not stop taking this medication unless directed by your doctor. If you are planning pregnancy, become pregnant, or think you may be pregnant, immediately discuss the benefits and risks of using this medication during pregnancy with your doctor.
This drug passes into breast milk and may have undesirable effects on a nursing infant. Consult your doctor before breast-feeding.
Interactions
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Some products that may interact with this drug include: other drugs that can cause bleeding/bruising (including antiplatelet drugs such as clopidogrel, NSAIDs such as ibuprofen/naproxen, "blood thinners" such as dabigatran/warfarin).
Aspirin can increase the risk of bleeding when used with this medication. However, if your doctor has directed you to take low-dose aspirin for heart attack or stroke prevention (usually 81-162 milligrams a day), you should continue taking it unless your doctor instructs you otherwise. Ask your doctor or pharmacist for more details.
Ask your doctor or pharmacist for more details.
Taking MAO inhibitors with this medication may cause a serious (possibly fatal) drug interaction. Avoid taking MAO inhibitors (isocarboxazid, linezolid, metaxalone, methylene blue, moclobemide, phenelzine, procarbazine, rasagiline, safinamide, selegiline, tranylcypromine) during treatment with this medication. Most MAO inhibitors should also not be taken for two weeks before and at least 7 days after treatment with this medication. Ask your doctor when to start or stop taking this medication.
The risk of serotonin syndrome/toxicity increases if you are also taking other drugs that increase serotonin. Examples include street drugs such as MDMA/"ecstasy," St. John's wort, certain antidepressants (including SSRIs such as fluoxetine/paroxetine, other SNRIs such as duloxetine/milnacipran), tryptophan, among others. The risk of serotonin syndrome/toxicity may be more likely when you start or increase the dose of these drugs.
Tell your doctor or pharmacist if you are taking other products that cause drowsiness such as opioid pain or cough relievers (such as codeine, hydrocodone), alcohol, marijuana (cannabis), drugs for sleep or anxiety (such as alprazolam, lorazepam, zolpidem), muscle relaxants (such as carisoprodol, cyclobenzaprine), or antihistamines (such as cetirizine, diphenhydramine).
Check the labels on all your medicines (such as allergy or cough-and-cold products) because they may contain ingredients that cause drowsiness. Ask your pharmacist about using those products safely.
Venlafaxine is very similar to desvenlafaxine. Do not take medications containing desvenlafaxine while using venlafaxine.
This medication may interfere with certain lab tests (including urine tests for amphetamines), possibly causing false test results. Make sure lab personnel and all your doctors know you use this drug.
Does Effexor XR interact with other drugs you are taking?
Enter your medication into the WebMD interaction checker
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include: severe drowsiness, seizures, fast/irregular heartbeat.
Symptoms of overdose may include: severe drowsiness, seizures, fast/irregular heartbeat.
Do not share this medication with others.
Keep all regular medical and psychiatric appointments. Laboratory and/or medical tests (such as blood pressure, cholesterol) should be performed periodically to monitor your progress or check for side effects. Consult your doctor for more details.
If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Images
Next
Related Links
Drug Survey
Are you currently using Effexor XR?
This survey is being conducted by the WebMD marketing sciences department.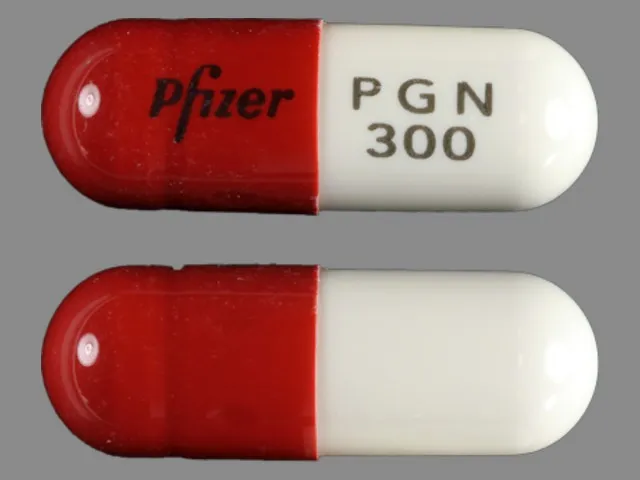
Free RX Coupon
Save up to 80% on your prescriptions.
Available coupons
Save up to 80% on your prescription with WebMDRx
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.
Venlafaxine Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing
Warnings:
Antidepressant medications are used to treat a variety of conditions, including depression and other mental/mood disorders. These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
Tell the doctor right away if you notice worsening depression/other psychiatric conditions, unusual behavior changes (including possible suicidal thoughts/attempts), or other mental/mood changes (including new/worsening anxiety, panic attacks, trouble sleeping, irritability, hostile/angry feelings, impulsive actions, severe restlessness, very rapid speech). Be especially watchful for these symptoms when a new antidepressant is started or when the dose is changed.
Warnings:
Antidepressant medications are used to treat a variety of conditions, including depression and other mental/mood disorders. These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
Tell the doctor right away if you notice worsening depression/other psychiatric conditions, unusual behavior changes (including possible suicidal thoughts/attempts), or other mental/mood changes (including new/worsening anxiety, panic attacks, trouble sleeping, irritability, hostile/angry feelings, impulsive actions, severe restlessness, very rapid speech). Be especially watchful for these symptoms when a new antidepressant is started or when the dose is changed.
. .. Show More
.. Show More
Uses
Venlafaxine is used to treat depression, anxiety, panic attacks, and social anxiety disorder (social phobia). It may improve your mood and energy level and may help restore your interest in daily living. It may also decrease fear, anxiety, unwanted thoughts, and the number of panic attacks. Venlafaxine is known as a serotonin-norepinephrine reuptake inhibitor (SNRI). It works by helping to restore the balance of certain natural substances (serotonin and norepinephrine) in the brain.
How to use Venlafaxine HCL ER
Read the Medication Guide and, if available, the Patient Information Leaflet provided by your pharmacist before you start using venlafaxine and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
Take this medication by mouth as directed by your doctor, usually once daily with food, either in the morning or evening.
Do not crush, chew, or dissolve this medication. Doing so can release all of the drug at once, increasing the risk of side effects. Swallow whole without crushing or chewing.
Swallow whole without crushing or chewing.
If you are taking the capsules, swallow them whole. If you have trouble swallowing the capsules whole, you may open the capsule and sprinkle the contents onto a spoonful of applesauce. Swallow all of the mixture right away without chewing. Drink a glass of water after each dose.
The dosage is based on your medical condition and response to treatment. To reduce your risk of side effects, your doctor may direct you to start this medication at a low dose and gradually increase your dose. Follow your doctor's instructions carefully. Take this medication regularly to get the most benefit from it. To help you remember, take it at the same time each day.
Keep taking this medication even if you feel well. Do not stop taking this medication without consulting your doctor. Some conditions may become worse when this drug is suddenly stopped. Also, you may experience symptoms such as confusion, mood swings, blurred vision, headache, tiredness, sleep changes, and brief feelings similar to electric shock. Your dose may need to be gradually decreased to reduce side effects. Report any new or worsening symptoms right away.
Your dose may need to be gradually decreased to reduce side effects. Report any new or worsening symptoms right away.
It may take several weeks to feel the benefit of this medication. Tell your doctor if your condition lasts or gets worse.
Side Effects
See also Warning section.
Nausea, drowsiness, dizziness, dry mouth, constipation, loss of appetite, blurred vision, nervousness, trouble sleeping, unusual sweating, or yawning may occur. If any of these effects last or get worse, tell your doctor promptly.
Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
This medication may raise your blood pressure. Check your blood pressure regularly and tell your doctor if the results are high.
Tell your doctor right away if you have any serious side effects, including: easy bruising/bleeding, decreased interest in sex, changes in sexual ability, muscle cramps/weakness, shaking (tremor).
Get medical help right away if you have any very serious side effects, including: cough that doesn't go away, shortness of breath, chest pain, severe/pounding headache, black/bloody stools, vomit that looks like coffee grounds, eye pain/swelling/redness, widened pupils, vision changes (such as seeing rainbows around lights at night), seizure.
This medication may increase serotonin and rarely cause a very serious condition called serotonin syndrome/toxicity. The risk increases if you are also taking other drugs that increase serotonin, so tell your doctor or pharmacist of all the drugs you take (see Drug Interactions section). Get medical help right away if you develop some of the following symptoms: fast heartbeat, hallucinations, loss of coordination, severe dizziness, severe nausea/vomiting/diarrhea, twitching muscles, unexplained fever, unusual agitation/restlessness.
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US - Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
Precautions
Before taking venlafaxine, tell your doctor or pharmacist if you are allergic to it; or to desvenlafaxine; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: bleeding problems, personal or family history of glaucoma (angle-closure type), high blood pressure, heart problems (such as heart failure, previous heart attack), high cholesterol, kidney disease, liver disease, seizure disorder, thyroid disease.
This drug may make you dizzy or drowsy or blur your vision. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. Do not drive, use machinery, or do anything that needs alertness or clear vision until you can do it safely. Avoid alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).
Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
Older adults may be more sensitive to the side effects of this drug, especially dizziness when standing. Older adults may also be more likely to develop a type of salt imbalance (hyponatremia), especially if they are taking "water pills" (diuretics). Dizziness and salt imbalance can increase the risk of falling. Older adults may also be at greater risk for bleeding while using this drug.
Children may be more sensitive to the side effects of the drug, especially loss of appetite and weight loss. Monitor weight and height in children who are taking this drug.
Monitor weight and height in children who are taking this drug.
During pregnancy, this medication should be used only when clearly needed. It may harm an unborn baby. Also, babies born to mothers who have used this drug during the last 3 months of pregnancy may rarely develop withdrawal symptoms such as feeding/breathing difficulties, seizures, muscle stiffness, or constant crying. If you notice any of these symptoms in your newborn, tell the doctor promptly.
Since untreated mental/mood problems (such as depression, anxiety, panic attacks) can be a serious condition, do not stop taking this medication unless directed by your doctor. If you are planning pregnancy, become pregnant, or think you may be pregnant, immediately discuss the benefits and risks of using this medication during pregnancy with your doctor.
This drug passes into breast milk and may have undesirable effects on a nursing infant. Consult your doctor before breast-feeding.
Interactions
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Some products that may interact with this drug include: other drugs that can cause bleeding/bruising (including antiplatelet drugs such as clopidogrel, NSAIDs such as ibuprofen/naproxen, "blood thinners" such as dabigatran/warfarin).
Aspirin can increase the risk of bleeding when used with this medication. However, if your doctor has directed you to take low-dose aspirin for heart attack or stroke prevention (usually 81-162 milligrams a day), you should continue taking it unless your doctor instructs you otherwise. Ask your doctor or pharmacist for more details.
Taking MAO inhibitors with this medication may cause a serious (possibly fatal) drug interaction. Avoid taking MAO inhibitors (isocarboxazid, linezolid, metaxalone, methylene blue, moclobemide, phenelzine, procarbazine, rasagiline, safinamide, selegiline, tranylcypromine) during treatment with this medication. Most MAO inhibitors should also not be taken for two weeks before and at least 7 days after treatment with this medication. Ask your doctor when to start or stop taking this medication.
Most MAO inhibitors should also not be taken for two weeks before and at least 7 days after treatment with this medication. Ask your doctor when to start or stop taking this medication.
The risk of serotonin syndrome/toxicity increases if you are also taking other drugs that increase serotonin. Examples include street drugs such as MDMA/"ecstasy," St. John's wort, certain antidepressants (including SSRIs such as fluoxetine/paroxetine, other SNRIs such as duloxetine/milnacipran), tryptophan, among others. The risk of serotonin syndrome/toxicity may be more likely when you start or increase the dose of these drugs.
Tell your doctor or pharmacist if you are taking other products that cause drowsiness such as opioid pain or cough relievers (such as codeine, hydrocodone), alcohol, marijuana (cannabis), drugs for sleep or anxiety (such as alprazolam, lorazepam, zolpidem), muscle relaxants (such as carisoprodol, cyclobenzaprine), or antihistamines (such as cetirizine, diphenhydramine).
Check the labels on all your medicines (such as allergy or cough-and-cold products) because they may contain ingredients that cause drowsiness. Ask your pharmacist about using those products safely.
Venlafaxine is very similar to desvenlafaxine. Do not take medications containing desvenlafaxine while using venlafaxine.
This medication may interfere with certain lab tests (including urine tests for amphetamines), possibly causing false test results. Make sure lab personnel and all your doctors know you use this drug.
Does Venlafaxine HCL ER interact with other drugs you are taking?
Enter your medication into the WebMD interaction checker
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include: severe drowsiness, seizures, fast/irregular heartbeat.
Symptoms of overdose may include: severe drowsiness, seizures, fast/irregular heartbeat.
Do not share this medication with others.
Keep all regular medical and psychiatric appointments. Laboratory and/or medical tests (such as blood pressure, cholesterol) should be performed periodically to monitor your progress or check for side effects. Consult your doctor for more details.
If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Images
Next
Related Links
Drug Survey
Are you currently using Venlafaxine HCL ER?
This survey is being conducted by the WebMD marketing sciences department.
Free RX Coupon
Save up to 80% on your prescriptions.
Available coupons
Save up to 80% on your prescription with WebMDRx
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.
Clinical efficacy and tolerability of venlafaxine (Velaxin) in the treatment of moderate and severe depression :: DIFFICULT PATIENT
S. N. Mosolov, E.G. Kostyukova, A.V. Gorodnichev, I.V. Timofeev, M.Ya. Ladyzhensky, O.V. Serditov
N. Mosolov, E.G. Kostyukova, A.V. Gorodnichev, I.V. Timofeev, M.Ya. Ladyzhensky, O.V. Serditov
Moscow Research Institute of Psychiatry, Roszdrav
The treatment of depressive spectrum disorders, from the 1960s to the present, remains a complex and urgent problem. Despite the variety of antidepressants available in the arsenal of psychiatrists, there is a large group of patients in whom known drugs are ineffective or cause undesirable effects. In this regard, studies of the pathogenesis of depression and the mechanisms of action of antidepressants are actively continuing, which makes it possible to synthesize new effective drugs with an improved tolerability profile and a more powerful mechanism of action.
Venlafaxine is the first third-generation thymoanaleptic (selective serotonin and norepinephrine reuptake inhibitor - SNRI), followed by a whole group of such drugs (milnacipran, duloxetine). It should be noted that venlafaxine is the most studied and most commonly prescribed drug from the SNRI group and is used in many studies (in order to prove the effectiveness of new antidepressants) as a reference.
In the last decade, convincing data have accumulated on the high effectiveness of tricyclic antidepressants (TCAs) compared with SSRIs in the treatment of severe, including melancholic depression. The results of two independent studies are known [1, 2], which compared the effectiveness of clomipramine, citalopram and paroxetine. The therapeutic response in treatment with clomipramine was 2 times higher than that of citalopram (60 and 30%). Similar results were noted when comparing clomipramine and paroxetine. The superiority of TCAs in the treatment of severe depressive states was also revealed in two meta-analyses conducted by Anderson [3, 4]. They confirmed that TCAs with a broad, non-selective mechanism of action (amitriptyline and clomipramine) are more effective than SSRIs. In our country, similar data were obtained by A.B. Smulevich and S.N. Mosolov on the material of open studies [5, 6].
The results of these studies, as well as clinical practice, led to the idea to enhance the effectiveness of antidepressants by expanding the neurochemical application, which became the basis for the synthesis of a new generation of antidepressants, namely: a wide range of clinical action; 2) compared to TCAs, dual action antidepressants are safer; 3) "dual" action antidepressants have a number of features and specific effects. These provisions, as shown by clinical practice, were fully realized in the process of using the "double" action antidepressant venlafaxine.
These provisions, as shown by clinical practice, were fully realized in the process of using the "double" action antidepressant venlafaxine.
Venlafaxine, having a wide therapeutic range of doses, consistently includes serotonergic, noradrenergic and dopaminergic effects in the spectrum of its neurochemical activity. So, at a dose of 75-125 mg, venlafaxine exhibits a serotonergic effect, when the dose is increased to 225 mg, the noradrenergic effect is turned on, and a further increase in the dose to 375 mg leads to the appearance of a dopaminergic effect. Such a dose-dependent pharmacology of venlafaxine fundamentally distinguishes this drug from SSRIs, a limited range of therapeutic doses, which narrows the possibility of other neurochemical effects other than serotonergic ones [7].
The high efficacy of venlafaxine has been demonstrated in comparative studies with TCAs. Thus, a comparison of venlafaxine and clomipramine did not reveal differences in the antidepressant effect, with slightly better tolerability of venlafaxine due to a lower frequency of anticholinergic adverse events (p ≤ 0. 05) [8]. Venlafaxine is as effective as amitriptyline [9] and imipramine, although the doses used in the study were close to the maximum (imipramine 179 mg/day versus venlafaxine 183 mg/day) [10].
05) [8]. Venlafaxine is as effective as amitriptyline [9] and imipramine, although the doses used in the study were close to the maximum (imipramine 179 mg/day versus venlafaxine 183 mg/day) [10].
A meta-analysis of 19 studies including 2181 patients showed that the side effects of venlafaxine, the frequency of which exceeds 10%, include: nausea, headache, insomnia, drowsiness, dry mouth, dizziness, constipation, asthenia, sweating and nervousness [ eleven]. Of these, nausea is the most common adverse event and leads to drug withdrawal in 6% of cases. At the same time, the frequency of occurrence of this undesirable effect decreases by 50% already after the 1st week of therapy and further - during the entire course of treatment. Anticholinergic side effects occur with venlafaxine 2 times less frequently than with TCAs.
However, some side effects of venlafaxine are noted to be dose-dependent (which is a direct reflection of the drug's dose-dependent pharmacology). These include with high certainty such serotonergic adverse events as nausea, insomnia and sexual dysfunctions [12].
The aim of this work was to study the efficacy, features of thymoanaleptic action and tolerability of the drug venlafaxine in the treatment of moderate and severe depression.
The study was open, non-comparative. The duration of the study was 6 weeks.
The study included patients aged 18 to 65 years with an ICD-10 diagnosis of recurrent depressive disorder (F32.1), moderate or severe depressive episode (F33.1 or F33.2), bipolar affective disorder (F31): episode moderate or severe depression (F31.3 and F31.4). The minimum sum of points when assessed on the 17-point Hamilton Scale (HH) was at least 17, and on the Global Clinical Impression Scale (GCI) - at least 4 points. The study included patients who gave written informed consent to participate in it. Quality of life was also assessed using the WHO Brief Quality of Life Scale at baseline and end of the study.
The exclusion criteria were the period of pregnancy and lactation in women; high suicidal risk for outpatients; any clinically significant uncompensated diseases of the kidneys, liver, cardiovascular, respiratory system, cerebrovascular disorders or other serious progressive somatic diseases; organic diseases of the central nervous system, any of the listed methods of treatment at the indicated time intervals before the start of the study: MAO inhibitors (including reversible ones) - 2 weeks; electroconvulsive therapy - 3 months; depot forms of neuroleptics - 4 weeks.
Patients taking any psychotropic medication prior to study entry were subject to a 7-day "wash out" period.
In the first two weeks of the study, including the "withdrawal" period, hypnotics were allowed to correct sleep disorders and anxiolytics (with the exception of alprazolam) for anxiety symptoms that could lead to premature termination of the study. Subsequently, this concomitant therapy was discontinued in all patients, with the exception of those who took benzodiazepine tranquilizers for a long time before the start of the study. No specific psychotherapy was used in the study. Patients were given explanations about their condition, including the expected effect of the drug.
During the follow-up, the severity of depressive symptoms was recorded using the Hamilton Scale for Depression (17 items) (HAM-D), the Global Clinical Impression (CGI) Scale, and the WHO Concise Quality of Life Scale. The condition of patients was assessed immediately before the start of the drug (day 0 of therapy), and then on days 4, 7, 14, 21, 28, 35, and 42 of treatment. On the same days, throughout the study, all emerging adverse events (AEs) were recorded with an indication of their severity, time of occurrence and duration. An AE was any adverse (medically) event that occurred in a patient receiving study drug, or regardless of its causal relationship to it. Worsening of the patient's pre-study symptoms was also recorded as an adverse event.
On the same days, throughout the study, all emerging adverse events (AEs) were recorded with an indication of their severity, time of occurrence and duration. An AE was any adverse (medically) event that occurred in a patient receiving study drug, or regardless of its causal relationship to it. Worsening of the patient's pre-study symptoms was also recorded as an adverse event.
The study included 30 patients, of which 26 (86.6%) women and 4 (13.4% men). The mean age of the patients was 43.6 ± 2.7 years.
The duration of the disease averaged 13.0 ± 1.9 years, the number of previous depressive episodes was 6.6 ± 1.2. The duration of the present episode of depression averaged 3.6 ± 1.22 months. Drug treatment of the current depressive episode before the start of the study was carried out in 13 (50%) patients.
Baseline mean group score for the first 17 HAM-D items was 21.96 ± 2.4. According to CGI, the average disease severity score for the group was 4.34 ± 0.48 points.
Venlafaxine (Velaxin) was given as a single daily dose of 75 mg/day followed by an increase to 150 mg/day in 2 divided doses during the first week. With insufficient effectiveness of the drug, the dose was increased to 225 mg / day. The dose increase was carried out 3 weeks after the start of therapy. The mean daily dose of venlafaxine was 137.5 mg/day.
With insufficient effectiveness of the drug, the dose was increased to 225 mg / day. The dose increase was carried out 3 weeks after the start of therapy. The mean daily dose of venlafaxine was 137.5 mg/day.
Completed the study 26 patients. One patient withdrew due to refusal to continue the study, 2 patients withdrew due to deterioration between days 14 and 21 of therapy, and 1 patient withdrew due to adverse events on day 3 of therapy.
Results of the study
When analyzing the data, the following results were obtained. The overall effectiveness of therapy by the end of the study for SH (the percentage of patients with a reduction in the total score by at least 50%) was 77% (20 out of 26 patients). Qualitative remission (≤ 7 points) by the end of the study was established in 61%. According to the CGI scale, a positive effect (pronounced and significant improvement) was observed in 18 (69%) patients.
Already at the second week of therapy, 40% of patients were assessed as responders to ongoing therapy, by the third week of therapy 50% were responders, and by the 6th week of treatment their number exceeded two-thirds of the total number (20 out of 27-74. 7%) . A significant increase in the number of responders by the 3rd week of therapy indicates a fairly rapid effect, somewhat ahead of the time characteristic of most antidepressants. From fig. Table 1 shows that the increase in the number of patients with a positive effect of therapy was observed until the end of the study, although it was most pronounced between the 3rd and 5th weeks of therapy, which suggests a fairly rapid onset of the effect in comparison with other new generation antidepressants, as well as Extend recommendations to continue therapy for at least 6 weeks for venlafaxine.
7%) . A significant increase in the number of responders by the 3rd week of therapy indicates a fairly rapid effect, somewhat ahead of the time characteristic of most antidepressants. From fig. Table 1 shows that the increase in the number of patients with a positive effect of therapy was observed until the end of the study, although it was most pronounced between the 3rd and 5th weeks of therapy, which suggests a fairly rapid onset of the effect in comparison with other new generation antidepressants, as well as Extend recommendations to continue therapy for at least 6 weeks for venlafaxine.
These data are also confirmed by the analysis of the dynamics of depression severity indicators according to CGI. A change in the severity of the disease was observed already on the 7th day of therapy (p The effect of therapy occurred quite quickly. The value of the total SH score for the group as a whole decreased by 12% already on the 4th day and by 23% on the 7th day of treatment ( the changes are statistically significant, p ≤ 0. 01) (Fig. 2). Subsequently, there was a consistent decrease in the severity of depressive symptoms. By the end of the study, the total score of SH was 6.9± 0.9 points, which practically corresponds to the generally accepted indicator of therapeutic remission for SH and indicates a distinct antidepressant effect of the study drug.
01) (Fig. 2). Subsequently, there was a consistent decrease in the severity of depressive symptoms. By the end of the study, the total score of SH was 6.9± 0.9 points, which practically corresponds to the generally accepted indicator of therapeutic remission for SH and indicates a distinct antidepressant effect of the study drug.
The main indicators that determine the spectrum of the psychotropic action of the antidepressant (low mood, mental anxiety and lethargy) decreased fairly evenly (Fig. 3), the statistical significance of changes for each indicator was observed already on the 7th day of therapy (p Despite a fairly harmonious reduction in anxiety and lethargy, in the first days the reduction of anxiety symptoms prevailed, but by the end of the first week of therapy, the reduction in indicators leveled off.0007 The nature of the thymoanaleptic effect is clearly confirmed by the analysis of the coefficient (K) of the ratio of anxiety reduction and lethargy (Fig. 4). The value of K, equal to 1, reflects a uniform reduction in the SH indicators "mental anxiety" and "lethargy" and indicates a balanced thymoanaleptic effect of the drug. As can be seen from fig. 4, at the beginning of therapy, the K value was significantly greater than 1, especially from the 4th day until the 21st day of therapy. This means that at the beginning of therapy, anxiety was reduced much faster than inhibition. In the study, this led to a rapid reduction in anxiety symptoms, the absence of the need for additional use of tranquilizers. However, by the 4th week of therapy, the effect of venlafaxine becomes balanced until the end of the course of therapy.
As can be seen from fig. 4, at the beginning of therapy, the K value was significantly greater than 1, especially from the 4th day until the 21st day of therapy. This means that at the beginning of therapy, anxiety was reduced much faster than inhibition. In the study, this led to a rapid reduction in anxiety symptoms, the absence of the need for additional use of tranquilizers. However, by the 4th week of therapy, the effect of venlafaxine becomes balanced until the end of the course of therapy.
It has already been mentioned above that venlafaxine, used in different doses, can have different effects on the reduction of depressive symptoms. Thus, venlafaxine at a dose of 75-150 mg / day affects mainly serotonergic transmission and is similar to SSRIs. The reduction in the anxiety/lethargy ratio for the group of patients who took venlafaxine at doses up to 150 mg/day is shown in Figure 5.
influence on lethargy, however, by the end of the first week, the action of venlafaxine became more balanced with an extremely insignificant activating effect after a month of therapy.
The effect of venlafaxine at a dose of more than 150 mg/day on the anxiety/lethargy reduction ratio is somewhat different (Fig. 6).
Noteworthy is the long-term, up to the 3rd week of therapy, the predominance of the reduction of anxiety symptoms in relation to the reduction of lethargy, which was assessed by patients as the absence of anxiety and did not require anxiolytics. Subsequently, until the end of the study, the drug prescribed at this dose was characterized as a balanced antidepressant with a mild sedative effect.
Clinically, at the end of the first week of treatment, most patients felt better. Along with a rapid decrease in the severity of anxiety, there was an improvement in sleep. Patients noted an improvement in the quality of sleep, it became deeper, a feeling of cheerfulness returned when waking up in the morning. The results are shown in Figure 7.
The rapid decrease in the severity of anxiety allowed most patients to stop taking anxiolytics prescribed during the "wash out" period or at previous stages of treatment. Objectively, patients became more contact, more active in conversation, noted that the interest lost during the period of depression in the usual range of activities and hobbies was beginning to be restored. These changes were reflected in the analysis of the WHO Brief Quality of Life Scale (Fig. 8).
Objectively, patients became more contact, more active in conversation, noted that the interest lost during the period of depression in the usual range of activities and hobbies was beginning to be restored. These changes were reflected in the analysis of the WHO Brief Quality of Life Scale (Fig. 8).
The results show a high reliability of improvement in this indicator (p ≤ 0.01) in patients after the course of therapy compared with the moment of inclusion in the study.
In the present study, venlafaxine appeared to be well tolerated. In one case, a patient was withdrawn from the study due to mydriasis. The adverse event was not severe, but the patient refused to continue therapy and dose adjustments. In 14 (43.3%) of 29 patients, 20 cases of AE were registered, however, the relationship of their development with venlafaxine was assessed as "probable" only in 6 cases, and in other cases as "supposed". Assessment of the intensity of AE showed that 12 (60%) of 20 cases of AE were of low intensity, 8 (40%) were moderate. In most cases, no measures were taken regarding therapy, but in 5 of 20 (40%) cases, the dose of the drug was reduced. All AEs that occurred during the study period and occurred with a frequency of more than 1 case are presented in the table.
In most cases, no measures were taken regarding therapy, but in 5 of 20 (40%) cases, the dose of the drug was reduced. All AEs that occurred during the study period and occurred with a frequency of more than 1 case are presented in the table.
As can be seen from the table, nausea and mydriasis occupy the largest share among AEs. In most cases, they appeared in the first two weeks of therapy. Drowsiness, as a rule, was observed on the 1st week of treatment, which, along with indicators of the dynamics of the ratio of anxiety reduction and lethargy, probably reflects some sedative effect of the drug in the first days of therapy. Analysis of the outcomes of AEs showed that in the vast majority of cases (80%) AEs were transient. In most cases, they developed in the first week of therapy when using a dose of 150 mg / day and, in the future, as therapy continued, they were reduced. With a further increase in the dose of the drug in most patients, an increase in the frequency of AE, as a rule, did not occur. In addition, after the first week of therapy, adverse events were noted only when using doses of 150 mg / day.
In addition, after the first week of therapy, adverse events were noted only when using doses of 150 mg / day.
Discussion
Summing up the results of the study, it is necessary to note the high efficacy of venlafaxine, and, most importantly, a 50% reduction in symptoms occurred fairly quickly, on the 2-3rd week of therapy. There are data in the literature on an earlier clinical response (within the 1st, rarely 2nd week of therapy) when using high doses of venlafaxine [13, 14, 15]. However, the design of these studies was based on the rapid buildup of venlafaxine. The most revealing study compared 3 groups of patients taking different doses of venlafaxine (75 mg - the first group; 150-225 mg - the second group; 300-375 mg - the third group). The results of this work showed that in the 1st and 2nd groups of patients, the onset of the primary response was observed in the first 2 weeks of therapy, while in the 3rd group of patients taking the highest dose of the drug, a statistically significant effect was noted already in the 1st week of treatment [15]. The dose escalation regimen in the present study differed from those described above in greater flexibility, which allowed the safe and effective use of venlafaxine for the treatment of depressive disorders in outpatients. It is clear that a rapid dose escalation strategy that allows full use of the dose-dependent effect of venlafaxine is especially appropriate in the treatment of major depressive disorders in an inpatient setting.
The dose escalation regimen in the present study differed from those described above in greater flexibility, which allowed the safe and effective use of venlafaxine for the treatment of depressive disorders in outpatients. It is clear that a rapid dose escalation strategy that allows full use of the dose-dependent effect of venlafaxine is especially appropriate in the treatment of major depressive disorders in an inpatient setting.
The dose-dependent effect of venlafaxine, associated with the gradual inclusion of various mediator structures (serotonin, norepinephrine, dopamine) into the orbit of the drug's influence, indirectly leads to a difference in the features of the clinical action. Thus, in the study, the drug used in high doses (more than 150 mg/day) proved to be predominantly sedative, while in smaller doses it had a more balanced effect.
The drug was quite well tolerated. None of the patients had severe adverse events, the vast majority of side effects were dose-dependent and were observed mainly in the first 2 weeks of therapy.
Given the rather pronounced sedative effect of the drug in the first weeks of therapy and the ability to control it depending on the dynamics of the dose, as well as the rapid reduction of sleep disturbances and the balanced nature of the action after 3 weeks of therapy, it can be noted that venlafaxine is one of the optimal drugs of choice for depressive patients on an outpatient basis.
Thus, summarizing the results of the study, we can conclude that venlafaxine has a distinct thymoanaleptic effect and has a balanced effect on the symptoms of anxiety and lethargy, with a moderate sedative effect in the first week of therapy. Of particular importance is the ability of the drug to quickly reduce depressive disorders of any severity, as well as a wide range of comorbid depression and anxiety disorders.
Literature.
1. Danish University Antidepressant Group. Citalopram: clinical effect profile in comparison with clomipramine. A controlled multicenter study // Psychopharmacology 1986; 90:131-138.
2. Danish University Antidepressant Group. Paroxetine: a selective serotonin reuptake inhibitor showing better tolerance, but weaker antidepressant effect than clomipramine in a controlled multicenter study // J Affective Disorders 1990; 18:289-299.
3. Anderson I.M., Tomenson B.M. The efficacy of selective serotonin re-uptake inhibitors in depression: a meta-analysis of studies against tricyclic antidepressants. J Psychopharmacol 1994; 8:238-49.
4. Anderson I.M. SSRIs versus tricyclic antidepressants in depressed inpatients: a meta-analysis of efficacy and tolerability // Depression Anxiety 1998; 7: Suppl. 1:11-17.
5. Mosolov S.N. Clinical use of modern antidepressants. St. Petersburg: 1995.
6. Smulevich A.B. Depression in general medicine. M.: 2001.
7. Preskorn S.H. Applied Clinical Psychopharmacology // J Practical Psychiatry and Behavioral Health, 1999, July; 224-8.
8. Samuelian J.C., Hackett D. A randomized, double-blind comparison of venlafaxine and clomipramine in outpatients with major depression // J Psychopharmacol 1998; 12:3:273-278.
9. Benedictis E. Double-blind comparison of venlafaxine and amitriptyline in outpatients with or without melancholia // J Psychopharmacol 2000; 14:1:61-66.
10. Schweizer E., Feigner J., Mandos et al. Comparison of venlafaxine and imipramine in the acute treatment of major depression in outpatients // J Clin Psychiatry 1994; 55:3:104-108.
11. Danjou P, Hackett D. Safety and tolerance profile of venlafaxine // Internat Clin Psychopharmacol 1995; 10:15-20.
12. Preskorn S.H. Comparison of the tolerability of buproprion, fluoxetine, imipramine, nefazodone, paroxetine, sertraline and venlafaxine // J Clin Psychiat 1995; 56: 1221.
13. Wang C.P., Howell S.R., Scatina J., Sisenwine S.F. The disposition of venlafaxine enantomers in dogs, rats, and humans receiving venlafaxine// Chirality 1992; 4:84-90.
14. Khan A., Fabre L., Rudolph R. Venlafaxine in depressed outpatients // Psychopharmacol Bull 1991; 27:141-4.
15. Schweizer E., Weise C., Clary C. et al. Placebo controlled trial of venlafaxine for the treatment of major depression // J Clin Psychopharmacol 1991; 11:233-236.
Placebo controlled trial of venlafaxine for the treatment of major depression // J Clin Psychopharmacol 1991; 11:233-236.
Side Effects of Effexor XR: What You Need to Know
Introduction
If you have certain mental disorders, your doctor may suggest Effexor XR (venlafaxine) as a treatment option.
Effexor XR is a prescription drug used in adults to treat:
- major depressive disorder
- generalized anxiety disorder
- social anxiety disorder
- panic disorder
Effexor XR helps relieve the symptoms of these conditions. The drug comes in the form of capsules that you take by mouth once a day. If Effexor XR works for you, your doctor will likely recommend that you take it long term.
Effexor XR is an extended release (XR) formulation, which means that it releases the active ingredient slowly over an extended period of time.
For more information about Effexor XR, including its uses, see this detailed product article.
Like all medicines, Effexor XR can cause mild or serious side effects. Keep reading to find out more.
What are the most common side effects of Effexor XR?
Some people may experience mild or severe side effects while taking Effexor XR. Examples of commonly reported Effexor XR side effects include:
- nausea
- feeling tired
- sweating*
- constipation
- sexual side effects
What are the mild side effects of Effexor XR?
Effexor XR may cause mild side effects in some people. Examples of mild side effects reported with Effexor XR include:
- nausea
- feeling tired
- sweating*
- constipation
- sexual side effects
- dry mouth
- unusual dreams
- loss of appetite
- headache
- weight gain or weight loss*
In most cases, these side effects should be temporary. And some are easy to deal with. But if you have any symptoms that continue or bother you, talk to your doctor or pharmacist. And don't stop using Effexor XR unless your doctor recommends it.
But if you have any symptoms that continue or bother you, talk to your doctor or pharmacist. And don't stop using Effexor XR unless your doctor recommends it.
Effexor XR may cause mild side effects other than those listed above. See the Effexor XR Treatment Guide for details.
After the Food and Drug Administration (FDA) approves a drug, it monitors the drug's side effects. If you would like to notify the FDA of a side effect you had with Effexor XR, visit MedWatch.
What are the serious side effects of Effexor XR?
In rare cases, some people may develop serious side effects from taking Effexor XR. Serious side effects reported with Effexor XR include:
- Suicidal thoughts or behavior *
- Serotonin syndrome
- High blood pressure †
- Unusual bleeding
- Problems with eyes, such as closed -angled glaucoma or hypomination
- Allergic reaction †
- seizures
- lung problems such as pneumonia
- high cholesterol
If you develop serious side effects while taking Effexor XR, call your doctor right away. If the side effects seem life-threatening or if you think you need emergency medical attention, call 9 right away11 or your local emergency number.
If the side effects seem life-threatening or if you think you need emergency medical attention, call 9 right away11 or your local emergency number.
Frequently Asked Questions About Effexor XR Side Effects
Get answers to some frequently asked questions about side effects of Effexor XR.
Does my risk of side effects increase in the first week of taking Effexor XR?
It is possible. In the first week of treatment with Effexor XR, you may experience more side effects. But not everyone will experience side effects, and side effects can be different for each person.
When you first start taking a new medicine, your body needs to get used to it. Thus, you may experience more side effects in the first week. It takes about 3 days for Effexor XR to reach a constant blood level. During this time, as your body adjusts, you may be at a higher risk of side effects.
The side effects you experience may also depend on your other medical conditions or other medicines you are taking. If you have questions about what to expect when you first start taking Effexor XR, talk to your doctor or pharmacist.
If you have questions about what to expect when you first start taking Effexor XR, talk to your doctor or pharmacist.
Are there any long-term side effects of Effexor XR?
Yes, long-term side effects of Effexor XR are possible. Examples include weight gain, weight loss, and eye problems such as angle-closure glaucoma.
It is possible that taking Effexor XR for a longer period of time may increase the risk of long-term side effects. But this is not the case for everyone, as Effexor XR side effects can vary from person to person.
If you are concerned about the long-term side effects of Effexor XR, talk to your doctor or pharmacist.
Do the side effects of Effexor XR change depending on the dose I take (37.5 mg, 75 mg or 150 mg)?
It is possible. You may be at an increased risk of side effects if you take a higher dose of Effexor XR. This is because there are more drugs in your body and they can have a greater effect on you.
Effexor XR is available in dosages of 37. 5 mg, 75 mg and 150 mg. In most cases, the maximum recommended dose of Effexor XR is 225 mg per day. In some cases, a doctor may prescribe a dose of up to 300 mg per day. But this is not an FDA-approved dose.
5 mg, 75 mg and 150 mg. In most cases, the maximum recommended dose of Effexor XR is 225 mg per day. In some cases, a doctor may prescribe a dose of up to 300 mg per day. But this is not an FDA-approved dose.
You should always take the dosage prescribed by your doctor. They will determine the best dosage for your needs.
If you experience side effects from Effexor XR, talk to your doctor. They may want to lower your dose to see if it helps reduce your side effects.
Will there be side effects if I miss or stop taking Effexor XR?
Yes, it is possible that skipping a dose of Effexor XR or stopping treatment abruptly could cause certain side effects.
In particular, abrupt discontinuation of treatment may lead to a withdrawal syndrome. Examples of the symptoms of the cancellation that may occur if you stop taking Effexor XR, include:
- Excitement or irritability
- Consecration
- Dizziness
- Headache
- insomnia (sleep problems)
- seizure 9,0002 If you miss a dose of Effexor XR, take it as soon as you remember.
 But if it's almost time for your next dose, skip the missed dose and take your next dose as usual. You should not take two doses of Effexor XR to make up for a missed dose. This may increase the risk of side effects.
But if it's almost time for your next dose, skip the missed dose and take your next dose as usual. You should not take two doses of Effexor XR to make up for a missed dose. This may increase the risk of side effects. If you are interested in stopping your Effexor XR treatment, talk to your doctor first. They will likely want to gradually reduce your dose so that you do not experience withdrawal symptoms.
In most cases, your doctor will decrease your dose by 75 milligrams per week until you stop taking the drug. But be sure to follow your doctor's advice to reduce the dose and stop treatment.
Because some withdrawal symptoms can be severe, you should not stop taking Effexor XR without first talking to your doctor. They can help you safely stop treatment.
How long do the side effects of Effexor XR last?
It depends. Some side effects, such as nausea, may occur when you first start taking Effexor XR, but may go away after a while. Other side effects, including decreased appetite and weight changes, may persist throughout treatment.

If you are concerned about certain side effects of Effexor XR, talk to your doctor. They can discuss your risk for these side effects and how long they may last if you experience them. Your doctor can also treat your side effects so they don't last as long.
Side effects explained
Find out more about some of the side effects Effexor XR may cause.
Weight gain or weight loss
Weight gain or loss may occur in people taking Effexor XR. But these were not the common side effects reported in the Effexor XR studies.
What can help
If you are concerned about any unexpected weight gain or loss you experience while taking Effexor XR, talk to your doctor. They can help determine what your next steps should be.
sweating
You may experience sweating during treatment with Effexor XR. Sweating was one of the most common side effects reported in studies by people taking Effexor XR.
What can help
If you're sweating more than usual while taking Effexor XR and it's bothering you, talk to your doctor.
 They may be able to recommend ways to reduce this side effect. In some cases, they may recommend another medication to treat your mental condition.
They may be able to recommend ways to reduce this side effect. In some cases, they may recommend another medication to treat your mental condition. Suicidal ideation and behavior in children and young people
Effexor XR has a warning about the risk of suicidal ideation and behavior in children and young people (aged 18 to 24 years). The boxed warning is the most serious warning from the Food and Drug Administration (FDA). It warns doctors and patients about drug side effects that can be dangerous.
All antidepressants contain this warning about suicidal thoughts and behavior. These side effects may occur during the first few months after starting treatment or with each dose increase or decrease.
It is important to note that Effexor XR is not approved for use in persons under 18 years of age.
What can help
It is important that you tell your doctor if you notice any new or worsening symptoms of depression or suicidal thoughts or behavior.
 These may include:
These may include: - suicidal thoughts or suicide attempts
- violence or aggression
- anxiety or panic attacks
- feeling restless or irritable
- insomnia (sleep problems)
- changes in behavior or mood
Also tell your doctor if you notice any problems with your eyes. These may include:
- changes in your vision
- eye pain
- redness or swelling in or around the eye
If you notice any of these symptoms, talk to your doctor. If the side effects seem life-threatening or if you think you need emergency medical attention, call 9 right away11 or your local emergency number.
If you are a young person taking Effexor XR, your doctor will likely monitor you more closely during treatment for any symptoms of depression and suicidal thoughts or behavior.
Suicide Prevention
If you think someone is at immediate risk of harming themselves or harming another person:
- Call 911 or your local emergency number.
- Stay with the person until help arrives.
- Remove all weapons, knives, medicines, and other items that could cause harm.
- Listen, but don't judge, argue, threaten or yell.
If you or someone you know is thinking about suicide, get help from a crisis or suicide prevention hotline. Call the National Suicide Prevention Hotline at 800-273-8255.
High blood pressure
Effexor may increase blood pressure. In clinical studies, some people who did not already have high blood pressure developed the condition after starting treatment with Effexor XR.
If you already have high blood pressure, Effexor XR may make it worse.
What can help
Before you start taking Effexor XR, tell your doctor about any blood pressure problems you have or if you are taking blood pressure medication.
If you have untreated high blood pressure, your doctor will likely want to treat it before starting treatment with Effexor XR.
 This is because the drug can also increase blood pressure, which may not be safe if your blood pressure is already high.
This is because the drug can also increase blood pressure, which may not be safe if your blood pressure is already high. Your doctor will also check and monitor your blood pressure throughout your treatment.
Allergic reaction
Like most medicines, Effexor XR may cause an allergic reaction in some people. But it is not clear if this side effect occurred in studies.
Symptoms may be mild or severe and may include:
- skin rash
- itching
- redness (temporary warmth, redness, or increased skin color)
- swelling under the skin, usually on the eyelids, lips, hands or feet
- swelling of the mouth, tongue, or throat that makes breathing difficult
What may help
If you have mild symptoms of an allergic reaction, such as a mild rash, call your doctor right away. They may suggest an over-the-counter oral antihistamine such as Benadryl (diphenhydramine) or a topical product such as hydrocortisone cream to relieve your symptoms.

If your doctor confirms that you had a mild allergic reaction to Effexor XR, they will decide whether you should continue using it.
If you have symptoms of a severe allergic reaction, such as swelling or difficulty breathing, call 911 or your local emergency number right away. These symptoms can be life threatening and require immediate medical attention.
If your doctor confirms that you have had a serious allergic reaction to Effexor XR, he may ask you to switch to another treatment.
Tracking side effects
Consider keeping a record of any side effects you experience during your Effexor XR treatment. You can then share this information with your doctor. This is especially helpful when you first start taking new medications or using a combination of treatments.
Your side effect notes may include things like:
- what dose of the drug you were taking when you had the side effect
- How soon after starting this dose did you experience a side effect
- What were your symptoms due to the side effect
- How did it affect your daily activities
- What other medications did you also take
- Any other information you think important
Taking notes and sharing them with your doctor will help him learn more about how the drug is affecting you.
 Your doctor may use this information to adjust your treatment plan if necessary.
Your doctor may use this information to adjust your treatment plan if necessary. Effexor XR Warnings
Before you start taking Effexor XR, talk to your doctor about any medical conditions you have or any medications you are taking. They can determine if this medicine may be a safe treatment option for you.
Detailed warnings for this drug are listed below.
Boxed Warning: Suicidal Thoughts and Behaviors in Children and Young Adults
Effexor XR has a boxed warning for suicidal thoughts and behaviors in children and young adults (aged 18 to 24 years). The boxed warning is the most serious warning from the Food and Drug Administration (FDA). It warns doctors and patients about drug side effects that can be dangerous.
If you are a young person taking Effexor XR, your doctor will likely monitor you more closely during treatment for any symptoms of depression and suicidal thoughts or behavior.
It is important to note that Effexor XR is not approved for use in persons under 18 years of age.
See the "Explanation of Side Effects" section above for more information.
Other warnings
Effexor XR may not be right for you if you have certain medical conditions or other health conditions. Talk to your doctor about your medical history before taking Effexor XR. The list below includes factors to consider.
High blood pressure or other heart problems. Tell your doctor if you have high blood pressure before taking Effexor XR. This drug can cause an even higher increase in blood pressure. Your doctor will check your blood pressure before you start treatment. If this is not well managed, your doctor will likely recommend treating your high blood pressure before you start taking Effexor XR. Even if your blood pressure is well controlled, your doctor may want to monitor you more closely during your treatment with Effexor XR. This is to ensure that your blood pressure does not get too high.
Allergic reaction. If you have had an allergic reaction to Effexor XR or any of its ingredients, you should not take Effexor XR.
 Ask your doctor which other medicines are best for you.
Ask your doctor which other medicines are best for you. Bipolar disorder or mania. Effexor XR may aggravate the symptoms of bipolar disorder or mania. If you have one of these conditions, your doctor may recommend a different medication to treat your condition, or may monitor you more closely during treatment.
Glaucoma. Effexor XR can cause an eye condition called angle-closure glaucoma. If you already have angle-closure glaucoma, Effexor XR may make your condition worse. Talk to your doctor about any eye conditions you have before taking Effexor XR.
Liver problems. Effexor XR may cause elevated liver enzymes, which may be a sign of liver disease. If you already have liver problems, Effexor XR may make your condition worse. Before starting treatment with Effexor XR, tell your doctor about any liver disease you have.
Kidney problems. Effexor XR is eliminated from the body through the kidneys.
 If your kidneys are not working properly, your body will not be able to get rid of the drug as quickly as it should. This can cause levels of the drug to build up in the body, which can increase the risk of side effects. If you have any kidney problems, talk to your doctor before taking Effexor XR. They can determine if it is safe for you to take this drug.
If your kidneys are not working properly, your body will not be able to get rid of the drug as quickly as it should. This can cause levels of the drug to build up in the body, which can increase the risk of side effects. If you have any kidney problems, talk to your doctor before taking Effexor XR. They can determine if it is safe for you to take this drug. Convulsions or convulsions. Effexor XR may increase the risk of seizures. If you have seizures or have had seizures or seizures before, Effexor XR may further increase your risk of seizures. Talk to your doctor about whether it is safe for you to take Effexor XR.
Low blood sodium. Effexor XR may decrease the amount of sodium in your blood. If you already have low sodium, this can be dangerous. Low sodium levels can cause confusion, fatigue, seizures, and even coma. If you have low blood sodium levels, or have already had one, tell your doctor before taking Effexor XR.
High cholesterol.
 Effexor XR may cause an increase in cholesterol levels. If you already have high cholesterol, this medication may make it worse. Talk to your doctor to see if this drug is right for you.
Effexor XR may cause an increase in cholesterol levels. If you already have high cholesterol, this medication may make it worse. Talk to your doctor to see if this drug is right for you. Bleeding problems. Effexor XR may increase the risk of bleeding. If you have any medical conditions that can also cause bleeding problems, or if you are taking medications that can affect your blood, tell your doctor before taking Effexor XR. They may recommend a different medication for you, or they may monitor you more closely throughout your treatment.
Drinking alcohol and Effexor XR
You should not drink alcohol while taking Effexor XR. Alcohol may increase the risk of some side effects from Effexor XR.
If you have concerns about not drinking alcohol while taking Effexor XR, talk to your doctor.
Pregnancy and breast-feeding while taking Effexor XR
Detailed information on the use of Effexor XR during pregnancy or breast-feeding is given below.

pregnancy
It is not known if it is safe to take Effexor XR during pregnancy. If you are pregnant or planning to become pregnant, talk to your doctor before taking Effexor XR.
Breastfeeding
Effexor XR is not recommended while breastfeeding. Effexor XR passes into breast milk and may affect a nursing baby. If you are currently breastfeeding or planning to breastfeed, talk to your doctor about your options.
What to ask your doctor
Effexor XR may be an effective treatment for certain mental illnesses. But some people may experience side effects when taking this drug. Most side effects are mild, but some can be serious.
Before taking Effexor XR, it is important to discuss possible side effects with your doctor so you know what to look out for. Here are some questions you can ask your doctor:
- How do I treat any side effects I experience?
- Do I have an increased risk of side effects due to my other medical conditions?
- What should I do if I get pregnant while taking Effexor XR?
Consider subscribing to the Drink-Drink depression or anxiety newsletter if you are taking Effexor XR for any of these conditions.

Ask a pharmacist
Q:
If I am taking a monoamine oxidase inhibitor (MAOI), how long do I have to wait between stopping the MAOI and starting treatment with Effexor XR before I have side effects?
Patient Anonymous
A:
A: If you are taking Effexor XR with an MAOI such as the antidepressant Nardil (phenelzine) or the antibiotic Zyvox (linezolid), you should not take these medicines for 7 days after stopping Effexor XR unless recommended by a doctor. It is also important that you stop taking MAOIs at least 2 weeks before starting Effexor XR treatment.
Always talk to your doctor before stopping any medication.
Melissa Badowski, PharmD, MPH, FCCP Answers represent the views of our medical experts. All content is for informational purposes only and should not be considered medical advice.
Registration data: Drink-Drink has made every effort to ensure that all information is accurate, complete and up to date.