Quetiapine fumarate dosage
Side Effects, dosage, uses, and more
- Quetiapine oral tablets are available as brand-name drugs and as generic drugs. Brand names: Seroquel and Seroquel XR.
- Quetiapine comes in two forms: immediate-release oral tablet and extended-release oral tablet. The immediate-release version is released into the bloodstream right away. The extended-release version is slowly released into your bloodstream over time.
- Both forms of quetiapine tablets are used to treat schizophrenia and bipolar disorder. The extended-release tablet is also used to treat major depression in combination with antidepressants.
Quetiapine is a prescription drug. It comes in the form of a tablet you take by mouth. There are two versions of the tablet. The immediate-release version is released into the bloodstream right away. The extended-release version is slowly released into your bloodstream over time.
Quetiapine is available as the brand-name drugs Seroquel (immediate-release tablet) and Seroquel XR (extended-release tablet). Both forms are also available as generic drugs. Generic drugs usually cost less than the brand-name version. In some cases, they may not be available in every strength or form as the brand-name drug.
Quetiapine may be used as part of a combination therapy. This means you may need to take it with other medications.
Why it’s used
Quetiapine oral tablet is used to treat the symptoms of schizophrenia, bipolar disorder, or depression.
Quetiapine can be used to treat symptoms in adults who have depressive episodes or manic episodes caused by bipolar I disorder. For these cases, it can be used alone or with the drugs lithium or divalproex. It can also be used with lithium or divalproex for long-term treatment of bipolar I disorder. Quetiapine can be used in children ages 10–17 years to treat manic episodes caused by bipolar I disorder.
For major depression, quetiapine is used as an add-on treatment for people already taking antidepressant drugs. It’s used when your doctor decides that one antidepressant alone is not enough to treat your depression.
How it works
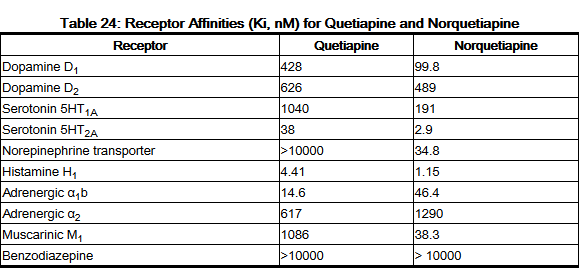
Quetiapine belongs to a class of drugs called atypical antipsychotics. A class of drugs is a group of medications that work in a similar way. These drugs are often used to treat similar conditions.
It isn’t known exactly how this drug works. However, it’s thought that it helps regulate the amount of certain chemicals (dopamine and serotonin) in your brain to control your condition.
Quetiapine oral tablet may cause drowsiness. It can also cause other side effects.
More common side effects
The side effects for this drug vary slightly based on the drug form.
The more common side effects of the immediate-release tablets can include:
- dry mouth
- dizziness
- pain in your stomach area
- constipation
- nausea
- vomiting
- weight gain
- increased appetite
- sore throat
- trouble moving
- rapid heartbeat
- weakness
The more common side effects of the extended-release tablets can include:
- dry mouth
- constipation
- dizziness
- increased appetite
- upset stomach
- tiredness
- stuffy nose
- trouble moving
If these effects are mild, they may go away within a few days or a couple of weeks. If they’re more severe or don’t go away, talk to your doctor or pharmacist.
If they’re more severe or don’t go away, talk to your doctor or pharmacist.
Serious side effects
Call your doctor right away if you have serious side effects. Call 911 if your symptoms feel life-threatening or if you think you’re having a medical emergency. Serious side effects and their symptoms can include the following:
- Suicidal thoughts or actions
- Neuroleptic malignant syndrome. Symptoms can include:
- high fever
- excessive sweating
- rigid muscles
- confusion
- changes in your breathing, heartbeat, and blood pressure
- Hyperglycemia (high blood sugar). Symptoms can include:
- extreme thirst
- frequent urination
- severe hunger
- weakness or tiredness
- upset stomach
- confusion
- fruity-smelling breath
- Increased cholesterol and triglycerides (high fat levels in your blood)
- Weight gain
- Tardive dyskinesia. Symptoms can include:
- movements you can’t control in your face, tongue, or other body parts
- Orthostatic hypotension (decreased blood pressure when rising too quickly after sitting or lying down).
 Symptoms can include:
Symptoms can include:- lightheadedness
- fainting
- dizziness
- Increases in blood pressure in children and teenagers
- Low white blood cell count. Symptoms can include:
- fever
- infection
- Cataracts. Symptoms can include:
- clouding of the lens of your eye
- blurry vision
- loss of vision
- Seizures
- Abnormal thyroid levels (shown in tests your doctor can do)
- Increases in blood prolactin levels. Symptoms can include:
- breast enlargement (in men and women)
- milky discharge from nipple of the breast (in women)
- erectile dysfunction
- absence of menstrual period
- Increased body temperature
- Trouble swallowing
- Risk of death from stroke in seniors with dementia
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs affect each person differently, we cannot guarantee that this information includes all possible side effects. This information is not a substitute for medical advice. Always discuss possible side effects with a healthcare provider who knows your medical history.
This information is not a substitute for medical advice. Always discuss possible side effects with a healthcare provider who knows your medical history.
Quetiapine oral tablet can interact with other medications, vitamins, or herbs you may be taking. An interaction is when a substance changes the way a drug works. This can be harmful or prevent the drug from working well.
To help avoid interactions, your doctor should manage all of your medications carefully. Be sure to tell your doctor about all medications, vitamins, or herbs you’re taking. To find out how this drug might interact with something else you’re taking, talk to your doctor or pharmacist.
Examples of drugs that can cause interactions with quetiapine are listed below.
Drugs you should not use with quetiapine
Do not take these drugs with quetiapine. Doing so can cause heart rhythm problems that could cause sudden death. Examples of these drugs include:
- Anti-arrhythmic drugs such as quinidine, procainamide, amiodarone or sotalol
- Antipsychotic drugs such as ziprasidone, chlorpromazine, or thioridazine
- Antibiotics such as gatifloxacin or moxifloxacin
- Pentamidine
- Methadone
Interactions that increase your risk of side effects
- Increased side effects from other drugs: Taking quetiapine with certain medications raises your risk of side effects from those drugs.
 Examples of these drugs include:
Examples of these drugs include:- Benzodiazepines such as alprazolam, clonazepam, diazepam, chlordiazepoxide or lorazepam. You may have increased drowsiness.
- Muscle relaxants such as baclofen, cyclobenzaprine, methocarbamol, tizanidine, carisoprodol, or metaxalone. You may have increased drowsiness.
- Pain medications such as morphine, oxycodone, fentanyl, hydrocodone, tramadol, or codeine. You may have increased drowsiness.
- Antihistamines such as hydroxyzine, diphenhydramine, chlorpheniramine, or brompheniramine. You may have increased drowsiness.
- Sedative/hypnotics such as zolpidem or eszopiclone. You may have increased drowsiness.
- Barbiturates such as phenobarbital. You may have increased drowsiness.
- Antihypertensives such as amlodipine, lisinopril, losartan, or metoprolol. Your blood pressure may be lowered even more.
- Increased side effects from quetiapine: Taking quetiapine with certain medications raises your risk of side effects from quetiapine. This is because the amount of quetiapine in your body may be increased. If you take these drugs with quetiapine, your doctor may decrease your quetiapine dosage. Examples of these drugs include:
- Antifungal drugs, such as ketoconazole or itraconazole
- HIV drugs such as indinavir or ritonavir
- Antidepressants such as nefazodone or fluoxetine
Using quetiapine with anticholinergic drugs may increase the risk of gastrointestinal problems.
Interactions that can make your drugs less effective
- When quetiapine is less effective: When quetiapine is used with certain drugs, it may not work as well to treat your condition. This is because the amount of quetiapine in your body may be decreased. If you take these drugs with quetiapine, your doctor may increase your quetiapine dosage.
 Examples of these drugs include:
Examples of these drugs include:- Anticonvulsants such as phenytoin or carbamazepine
- Rifampin
- St. John’s wort
- When other drugs are less effective: When certain drugs are used with quetiapine, they may not work as well. Examples of these drugs include:
- Parkinson’s disease medications such as levodopa, pramipexole, or ropinirole. Quetiapine may block the effects of your Parkinson’s medications. This may cause an increase in your symptoms of Parkinson’s disease.
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs interact differently in each person, we cannot guarantee that this information includes all possible interactions. This information is not a substitute for medical advice. Always speak with your healthcare provider about possible interactions with all prescription drugs, vitamins, herbs and supplements, and over-the-counter drugs that you are taking.
This drug comes with several warnings.
Neuroleptic malignant syndrome (NMS) warning
NMS is a rare but very serious condition that can occur in people who take antipsychotic drugs such as quetiapine. NMS can cause death and must be treated in a hospital. Symptoms can include high fever, excessive sweating, rigid muscles, confusion, or changes in breathing, heartbeat, or blood pressure. If you become very ill with these symptoms, call 911 right away.
Metabolic changes warning
Quetiapine can cause changes in the way your body functions. You may have hyperglycemia (high blood sugar), increased cholesterol and triglycerides (fats in the blood), or weight gain. High blood sugar can occur in people with or without diabetes. Symptoms can include feeling very thirsty or hungry, needing to urinate more than usual, feeling weak or tired, or having fruity-smelling breath. Your doctor will monitor you for these metabolic changes.
Tardive dyskinesia warning
Quetiapine can cause tardive dyskinesia. This is a serious condition that causes movements of the face, tongue, or other body parts that you can’t control. Tardive dyskinesia may not go away even if you stop taking quetiapine. It may also start after you stop taking this drug.
This is a serious condition that causes movements of the face, tongue, or other body parts that you can’t control. Tardive dyskinesia may not go away even if you stop taking quetiapine. It may also start after you stop taking this drug.
Anticholinergic effects
Taking quetiapine with other anticholinergic drugs may increase your risks of anticholinergic effects that may cause serious injuries. Symptoms may include severe constipation or stomach pain, inability to empty your bladder (urinary retention), blurred vision, drowsiness, delirium, confusion, and falls. Your risks may be increased if you are over 65 and have a history of these side effects. If you experience any of these symptoms, call your doctor right away.
Allergy warning
Quetiapine can cause a severe allergic reaction. Symptoms can include:
- trouble breathing
- swelling of your throat or tongue
If you develop these symptoms, call 911 or go to the nearest emergency room.-Tab-50mg-UK-1.jpg)
Don’t take this drug again if you’ve ever had an allergic reaction to it. Taking it again could be fatal (cause death).
Alcohol interaction warning
Quetiapine can cause drowsiness. The use of drinks that contain alcohol raises your risk of this side effect. If you drink alcohol, talk to your doctor about whether this drug is safe for you.
Warnings for people with certain health conditions
For people with diabetes or high blood sugar: Quetiapine may increase your blood sugar levels, which can worsen your condition. Extremely high blood sugar may lead to coma or death. If you have diabetes or risk factors of diabetes, talk with your doctor. They should check your blood sugar before and during treatment with quetiapine.
For people with hyperlipidemia (high fat levels in the blood): Quetiapine may further increase the levels of fat (cholesterol and triglycerides) in your blood. High fat levels raise your risk of heart attack and stroke. These high levels typically don’t cause symptoms. Therefore, your doctor may check your blood cholesterol and triglycerides during treatment with quetiapine.
These high levels typically don’t cause symptoms. Therefore, your doctor may check your blood cholesterol and triglycerides during treatment with quetiapine.
For people with low or high blood pressure: Quetiapine may worsen your high or low blood pressure. It may also increase blood pressure in children and teenagers. Your doctor should monitor your blood pressure while you take quetiapine.
For people with a low white blood cell count: Quetiapine may lower your low white blood cell count even more. Your doctor should monitor your white blood cell count often during your first few months of treatment. This can help make sure that quetiapine is not decreasing your white blood cell count.
For people with cataracts: Quetiapine may worsen your cataracts. Your doctor will monitor you for changes in your cataracts. They will examine your eyes when you start treatment and every 6 months during treatment.
For people with seizures: Seizures have occurred in patients with or without epilepsy while taking quetiapine. Quetiapine may make it harder to control seizures in people with epilepsy. Your doctor should monitor you for an increase in seizures while taking this drug.
Quetiapine may make it harder to control seizures in people with epilepsy. Your doctor should monitor you for an increase in seizures while taking this drug.
For people with hypothyroidism (low thyroid level): Quetiapine may lower thyroid hormone levels and worsen your existing condition. Your doctor should monitor your blood thyroid hormone levels before and during treatment with this drug.
For people with heart problems: Ask your doctor if this drug is safe for you. This drug increases the risk of abnormal heart rhythms.
For people with liver problems: Quetiapine is mainly broken down in the body by the liver. As a result, people with liver problems may have increased blood levels of this drug. This raises the risk of side effects from this drug.
For people with severe constipation: Quetiapine can cause constipation and increase your risk of gastrointestinal problems including bowel block. This has been deadly in people taking this drug with other drugs that slow movement through your gastrointestinal tract. If you have questions, talk with your doctor. Using quetiapine with anticholinergic drugs may increase the risk of gastrointestinal problems.
If you have questions, talk with your doctor. Using quetiapine with anticholinergic drugs may increase the risk of gastrointestinal problems.
Warnings for other groups
For pregnant women: Quetiapine is a category C pregnancy drug. That means two things:
- Research in animals has shown adverse effects to the fetus when the mother takes the drug.
- There haven’t been enough studies done in humans to be certain how the drug might affect the fetus.
Talk to your doctor if you’re pregnant or planning to become pregnant. This drug should only be used if the potential benefit justifies the potential risk.
For women who are breastfeeding: Quetiapine may pass into breast milk and may cause side effects in a child who is breastfed. Talk to your doctor if you breastfeed your child. You may need to decide whether to stop breastfeeding or stop taking this medication.
For seniors: The kidneys and livers of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
For children:
- Schizophrenia
- Episodes: This medication hasn’t been studied in children for this purpose. It shouldn’t be used in children younger than 13 years.
- Bipolar I mania
- Episodes: This medication hasn’t been studied in children for this purpose. It shouldn’t be used in children younger than 10 years.
- Bipolar disorder, depressive episodes: This medication hasn’t been studied in children for this purpose. It shouldn’t be used in children younger than 18 years.
- Major depressive disorder treated with antidepressants: This medication hasn’t been studied in children for this purpose. It shouldn’t be used in children younger than 18 years.

All possible dosages and drug forms may not be included here. Your dosage, drug form, and how often you take the drug will depend on:
- your age
- the condition being treated
- the severity of your condition
- other medical conditions you have
- how you react to the first dose
Drug forms and strengths
Generic: Quetiapine
- Form: immediate-release oral tablet
- Strengths: 25 mg, 50 mg, 100 mg, 200 mg, 300 mg, and 400 mg
- Form: extended-release oral tablet
- Strengths: 50 mg, 150 mg, 200 mg, 300 mg, and 400 mg
Brand: Seroquel
- Form: immediate-release oral tablet
- Strengths: 25 mg, 50 mg, 100 mg, 200 mg, 300 mg, and 400 mg
Brand: Seroquel XR
- Form: extended-release oral tablet
- Strengths: 50 mg, 150 mg, 200 mg, 300 mg, and 400 mg
Dosage for schizophrenia
Adult dosage (ages 18–64 years)
Immediate-release tablets
- Typical starting dosage:
- Day 1: 25 mg twice daily.

- Days 2 and 3: Your doctor will increase your dose by 25–50 mg. The total dosage should be taken two or three times daily.
- Day 4: 300–400 mg daily, taken in 2 or 3 divided doses.
- Day 1: 25 mg twice daily.
- Dosage increases:
- Your doctor may further increase your dosage not more often than every two days. The increase would be 25–50 mg added to your previous dosage. The total dosage would be taken twice daily.
- The recommended dosage range is 150–750 mg per day.
- Maintenance dosage: Your doctor may keep you on this medication to help control symptoms on an ongoing basis. The dosage range for maintenance use is 400–800 mg per day, taken in 2 or 3 divided doses.
- Maximum dosage: 800 mg per day, taken in 2 or 3 divided doses.
Extended-release tablets
- Typical starting dosage: 300 mg once per day.
- Dosage increases: Your doctor may increase your dosage every day by no more than 300 mg once per day.
 The recommended dosage range is 400–800 mg once per day.
The recommended dosage range is 400–800 mg once per day. - Maximum dosage: 800 mg per day.
Senior dosage (ages 65 years and older)
Your doctor may start you on a lowered dosage or a different dosing schedule. This can help keep levels of this drug from building up too much in your body. Your doctor may start you at a dosage of 50 mg daily. They may later increase it, adding 50 mg to your daily dose. The dosage may be increased at a slower rate, and a lower total daily dose may be used to lessen the risk of side effects.
Child dosage (ages 0–17 years)
SCHIZOPHRENIA EPISODES
Child dosage (ages 13–17 years)
Immediate-release tablets
- Typical starting dosage:
- Day 1: 25 mg twice daily.
- Day 2: 100 mg per day, taken in divided doses twice daily.
- Day 3: 200 mg per day, taken in divided doses twice daily.

- Day 4: 300 mg per day, taken in divided doses twice daily.
- Day 5: 400 mg per day, taken in divided doses twice daily.
- Dosage increases: Your doctor may further increase your child’s dosage by no more than 100 mg per day. The recommended dosage range is 400–800 mg per day, taken in 2 or 3 divided doses.
- Maximum dosage: 800 mg per day, taken in 2 or 3 divided doses.
Extended-release tablets
Typical starting dosage:
- Day 1: 50 mg once daily.
- Day 2: 100 mg once daily.
- Day 3: 200 mg once daily.
- Day 4: 300 mg once daily.
- Day 5: 400 mg once daily.
Child dosage (ages 0–12 years)
It has not been confirmed that quetiapine is safe and effective to use for this purpose in children younger than 13 years.
SCHIZOPHRENIA MAINTENANCE
Child dosage (ages 0–17 years)
This medication has not been studied in children to use for this purpose. It should not be used in children younger than 18 years.
It should not be used in children younger than 18 years.
Dosage for bipolar I disorder (manic or mixed episodes)
Adult dosage (ages 18–64 years)
Immediate-release tablets
- Typical starting dosage:
- Day 1: 100 mg per day, taken in divided doses twice daily.
- Day 2: 200 mg per day, taken in divided doses twice daily.
- Day 3: 300 mg per day, taken in divided doses twice daily.
- Day 4: 400 mg per day, taken in divided doses twice daily.
- Dosage increases: Your doctor may further increase your dosage by no more than 200 mg per day.
- Maintenance dosage: Your doctor may keep you on this medication to help control symptoms on an ongoing basis. The dosage range for maintenance use is 400–800 mg per day, taken in 2 or 3 divided doses.
- Maximum dosage: 800 mg per day, taken in 2 or 3 divided doses.
Extended-release tablets
- Typical starting dosage:
- Day 1: 300 mg once per day.

- Day 2: 600 mg once per day.
- Day 3: 400–800 mg once per day.
- Day 1: 300 mg once per day.
- Dosage increases: Your doctor may change your dosage within the recommended range of 400–800 mg once per day.
- Maximum dosage: 800 mg once per day.
Senior dosage (ages 65 years and older)
Your doctor may start you on a lowered dose or a different dosing schedule. This can help keep levels of this drug from building up too much in your body. Your doctor may start you at a dosage of 50 mg daily. They may later increase it, adding 50 mg to your daily dose. The dosage may be increased at a slower rate, and a lower total daily dose may be used to lessen the risk of side effects.
Child dosage (ages 10–17 years)
Immediate-release tablets
- Typical starting dosage:
- Day 1: 25 mg twice daily.
- Day 2: 100 mg per day, taken in divided doses twice daily.

- Day 3: 200 mg per day, taken in divided doses twice daily.
- Day 4: 300 mg per day, taken in divided doses twice daily.
- Day 5: 400 mg per day, taken in divided doses twice daily.
- Dosage increases: Your doctor may further increase your dose by no more than 100 mg per day. The recommended dosage range is 400–600 mg per day taken in divided doses up to three times daily.
- Maximum dosage: 600 mg per day in 2 or 3 divided doses.
Extended-release tablets
- Typical starting dosage:
- Day 1: 50 mg once per day.
- Day 2: 100 mg once per day.
- Day 3: 200 mg once per day.
- Day 4: 300 mg once per day.
- Day 5: 400 mg once per day.
- Dosage increases: Your doctor may change your dose, within the recommended dosage range of 400–600 mg once per day.
- Maximum dosage: 600 mg once per day.

Child dosage (ages 0–9 years)
It hasn’t been confirmed that quetiapine is safe and effective to use for this purpose in children younger than 10 years.
Dosage for bipolar I disorder (maintenance)
Child dosage (ages 0–17 years)
It hasn’t been confirmed that quetiapine is safe and effective to use for this purpose in children younger than 18 years.
Dosage for bipolar disorder (depressive episodes)
Adult dosage (ages 18–64 years)
Immediate-release tablets
- Typical starting dosage:
- Day 1: 50 mg daily, taken at bedtime.
- Day 2: 100 mg daily, taken at bedtime.
- Day 3: 200 mg daily, taken at bedtime.
- Day 4: 300 mg daily, taken at bedtime.
- Maximum dosage: 300 mg daily, taken at bedtime.
Extended-release tablets
- Typical starting dosage:
- Day 1: 50 mg once daily at bedtime.

- Day 2: 100 mg once daily at bedtime.
- Day 3: 200 mg once daily at bedtime.
- Day 4: 300 mg once daily at bedtime.
- Day 1: 50 mg once daily at bedtime.
- Maximum dosage: 300 mg once daily at bedtime.
Senior dosage (ages 65 years and older)
Your doctor may start you on a lowered dose or a different dosing schedule. This can help keep levels of this drug from building up too much in your body. Your doctor may start you at a dosage of 50 mg daily. They may later increase it, adding 50 mg to your daily dose. The dosage may be increased at a slower rate, and a lower total daily dose may be used to lessen the risk of side effects.
Child dosage (ages 0–17 years)
It hasn’t been confirmed that quetiapine is safe and effective to use for this purpose in children younger than 18 years.
Dosage for major depression in people already taking antidepressants
Extended-release tablets
Adult dosage (ages 18–64 years)
- Typical starting dosage:
- Days 1 and 2: 50 mg once daily.

- Day 3: 150 mg once daily.
- Days 1 and 2: 50 mg once daily.
- Dosage increases: Your doctor may change your dosage, within the recommended range of 150–300 mg once per day.
- Maximum dosage: 300 mg once daily.
Senior dosage (ages 65 years and older)
Your doctor may start you on a lowered dose or a different dosing schedule. This can help keep levels of this drug from building up too much in your body. Your doctor may start you at a dosage of 50 mg daily. They may later increase it, adding 50 mg to your daily dose. The dosage may be increased at a slower rate, and a lower total daily dose may be used to lessen the risk of side effects.
Child dosage (ages 0–17 years)
It hasn’t been confirmed that quetiapine is safe and effective to use for this purpose in children younger than 18 years.
Special dosage considerations
- For people with liver disease: Your doctor should start your dosage at 25 mg daily.
 This dosage may be increased by 25–50 mg daily.
This dosage may be increased by 25–50 mg daily. - Use with drugs called CYP3A4 inhibitors: Quetiapine dosage should be decreased to one-sixth of the original dosage when given with certain drugs called CYP3A4 inhibitors. Ask your doctor or pharmacist if you’re taking a CYP3A4 inhibitor. Examples of these drugs include ketoconazole, itraconazole, indinavir, ritonavir, or nefazodone. When the CYP3A4 inhibitor is stopped, the dose of quetiapine should be increased by 6 times the previous dose.
- Use with drugs called CYP3A4 inducers: Quetiapine dose should be increased by five times the original dose when given with certain drugs called CYP3A4 inducers. Ask your doctor or pharmacist if you’re taking a CYP3A4 inducer. Examples of these drugs include phenytoin, carbamazepine, rifampin, or St. John’s wort. When the CYP3A4 inducer is stopped, the dose of quetiapine should be reduced to the original dose within 7–14 days.
Dosage warnings
If you have stopped quetiapine for more than one week, you’ll need to be restarted at a lower dosage. The dosage will then need to be increased according to the dosage schedule from when you first started the medication.
The dosage will then need to be increased according to the dosage schedule from when you first started the medication.
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs affect each person differently, we cannot guarantee that this list includes all possible dosages. This information is not a substitute for medical advice. Always speak with your doctor or pharmacist about dosages that are right for you.
Quetiapine oral tablet is used for long-term treatment. It comes with serious risks if you don’t take it as prescribed.
If you stop taking the drug suddenly or don’t take it at all: Your condition may get worse. If you stop taking quetiapine suddenly, you may also have trouble sleeping or trouble staying asleep, or have nausea or vomiting.
If you miss doses or don’t take the drug on schedule: Your medication may not work as well or may stop working completely. For this drug to work well, a certain amount needs to be in your body at all times.
For this drug to work well, a certain amount needs to be in your body at all times.
If you take too much: You could have dangerous levels of the drug in your body. Symptoms of an overdose of this drug can include:
- drowsiness
- sleepiness
- fast heartbeat (palpitations)
- dizziness
- fainting
If you think you’ve taken too much of this drug, call your doctor or seek guidance from the American Association of Poison Control Centers at 1-800-222-1222 or through their online tool. But if your symptoms are severe, call 911 or go to the nearest emergency room right away.
What to do if you miss a dose: Take your dose as soon as you remember. But if you remember just a few hours before your next scheduled dose, take only one dose. Never try to catch up by taking two doses at once. This could result in dangerous side effects.
How to tell if the drug is working: Your behavior or mood should improve.
Keep these considerations in mind if your doctor prescribes quetiapine for you.
General
- You can take the immediate-release tablet with or without food. You should take the extended-release tablet without food or with a light meal (about 300 calories).
- Take this drug at the time(s) recommended by your doctor.
- You can cut or crush quetiapine immediate-release tablets. However, you can’t cut or crush quetiapine extended-release tablets.
Storage
- Store quetiapine at room temperature between 59°F and 86°F (15°C and 30°C).
- Keep this drug away from light.
- Don’t store this medication in moist or damp areas, such as bathrooms.
Refills
A prescription for this medication is refillable. You should not need a new prescription for this medication to be refilled. Your doctor will write the number of refills authorized on your prescription.
Travel
When traveling with your medication:
- Always carry your medication with you.
 When flying, never put it into a checked bag. Keep it in your carry-on bag.
When flying, never put it into a checked bag. Keep it in your carry-on bag. - Don’t worry about airport X-ray machines. They can’t harm your medication.
- You may need to show airport staff the pharmacy label for your medication. Always carry the original prescription-labeled container with you.
- Don’t put this medication in your car’s glove compartment or leave it in the car. Be sure to avoid doing this when the weather is very hot or very cold.
Self-management
Quetiapine can make your body less able to manage your temperature. This can cause your temperature to increase too much, leading to a condition called hyperthermia. Symptoms can include hot skin, excessive sweating, fast heartbeat, rapid breathing, and even seizures. To help prevent this, do the following during your treatment with this drug:
- Avoid getting overheated or dehydrated. Don’t over-exercise.
- During hot weather, stay inside in a cool place if possible.
- Stay out of the sun.
 Don’t wear heavy clothing.
Don’t wear heavy clothing. - Drink plenty of water.
Clinical monitoring
You and your doctor should monitor certain health issues. This can help make sure you stay safe while taking this drug. These issues include:
- Blood sugar. Quetiapine may raise your blood sugar level. Your doctor may monitor your blood sugar from time to time, especially if you have diabetes or are at risk of diabetes.
- Cholesterol. Quetiapine may increase the levels of fats (cholesterol and triglycerides) in your blood. You may not have symptoms, so your doctor may check your blood cholesterol and triglycerides at the start of treatment and during treatment with quetiapine.
- Weight. Weight gain is common in people who take quetiapine. You and your doctor should check your weight regularly.
- Mental health and behavioral problems. You and your doctor should watch for any unusual changes in your behavior and mood.
 This drug can cause new mental health and behavior problems, or worsen problems you already have.
This drug can cause new mental health and behavior problems, or worsen problems you already have. - Thyroid hormone levels. Quetiapine can decrease your thyroid hormone levels. Your doctor should monitor your thyroid hormone levels before starting treatment and throughout treatment with quetiapine.
Hidden costs
You may need to have blood tests from time to time to check your blood sugar and cholesterol levels. The cost of these tests will depend on your insurance coverage.
Prior authorization
Many insurance companies require a prior authorization for this drug. This means your doctor will need to get approval from your insurance company before your insurance company will pay for the prescription.
There are other drugs available to treat your condition. Some may be better suited for you than others. Talk to your doctor about other drug options that may work for you.
Disclaimer: Medical News Today has made every effort to make certain that all information is factually correct, comprehensive, and up-to-date. However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or other healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or other healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
How and when to take quetiapine
Dosage and strength
Standard quetiapine tablets come in strengths of 25mg, 100mg, 150mg, 200mg and 300mg.
The slow release tablets come in strengths of 50mg, 150mg, 200mg, 300mg, 400mg and 600mg.
The liquid contains 20mg of quetiapine in 1ml.-Tab-25mg-UK-2.jpg)
How much quetiapine you take depends on why you're taking it.
Your doctor may ask you to change your dose, depending on how well quetiapine works for you. It can take a few weeks to get to the dose that works best for you.
Doses may be lower for older people and people with liver problems.
Dose for schizophrenia
You'll usually start on a low dose of 50mg a day when taking standard tablets. This will be increased over a few days to start with.
Your doctor may advise you to slowly increase your dose even more, depending on how well quetiapine works for you. Most people feel better with a daily dose of 300mg to 600mg.
If you're taking standard tablets, take half your dose in the morning and half in the evening, unless your doctor gives you different instructions. If you're taking slow release tablets, take the whole daily amount in one dose.-Tab-4mg-Turkey-UK-1.jpg)
Dose for mania symptoms of bipolar disorder
You'll usually start on a low dose of 100mg a day when taking standard tablets. This will be increased over a few days to start with.
Your doctor may ask you to slowly increase your dose even more, depending on how well quetiapine works for you. Most people feel better with a daily dose of 400mg to 800mg.
If you're taking standard tablets, take half your dose in the morning and half in the evening, unless your doctor gives you different instructions. If you're taking slow release tablets, take the whole daily amount in one dose.
Dose for depression in bipolar disorder
You'll usually start on a low dose of 50mg a day. This will be increased over a few days. Most people feel better with a daily dose of 300mg.
Take it once a day at bedtime.
Dose for preventing mania or depression in bipolar disorder
If you've taken quetiapine to treat bipolar disorder and it has worked for you, you'll usually continue to take the same dose.
You doctor may ask you to increase or decrease your dose to find the best dose for you. Most people continue to feel better with a daily dose of 300mg to 800mg.
If you're taking standard tablets, take half your dose in the morning and half in the evening, unless your doctor gives you different instructions. If you're taking slow release tablets, take the whole daily amount in one dose.
Dose for depression when used with other medicines
You'll usually start on a low dose of 50mg. This will be increased over a few days. Most people feel better with a daily dose of 150mg to 300mg.
Take it once a day at bedtime.
How to take tablets
If you're taking standard tablets, swallow them whole with a drink of water. You can take them with or without food.
If you're taking slow release tablets, swallow your tablet whole with a drink of water without food. It's best to take the tablets at least 1 hour before a meal and at least 2 hours after a meal. Do not break, chew or crush the tablets.
It's best to take the tablets at least 1 hour before a meal and at least 2 hours after a meal. Do not break, chew or crush the tablets.
How to take liquid
If you're taking liquid quetiapine, it will come with a plastic syringe or dosing cup to help you measure out your dose. If you do not have one, ask your pharmacist for one. Do not use a kitchen spoon as it will not measure the right amount.
If your quetiapine liquid comes in a bottle with a syringe:
- Shake the bottle.
- Remove the cap and push the syringe adaptor into the bottle neck.
- Put the syringe into the opening in the adaptor and turn the bottle upside down.
- Fill the syringe to the right dose, turn the bottle the right way up and remove the syringe.
- Put the end of the syringe into your mouth, and push the plunger to squirt the medicine into your mouth.
 Swallow your dose straight away. Have a drink of water afterwards if you need to.
Swallow your dose straight away. Have a drink of water afterwards if you need to. - Wash the syringe and let it dry before you use it again.
To use the dosing cup, shake the bottle, then pour the liquid into the cup until it reaches the mark shown for your dose.
Wash the cup and let it dry before you use it again.
You can take the liquid with or without food.
How long to take it for
If you take quetiapine for schizophrenia or depression, you may need to take it for a long time, maybe several years.
If you take it for mania or depression in bipolar disorder, you may need to take it for a few weeks or months. Your doctor may suggest that you keep taking it for a long time, maybe several years, to stop your symptoms coming back.
Important
Keep taking quetiapine even if you feel better. Do not stop taking it without talking to your doctor.
Do not stop taking it without talking to your doctor.
If you forget to take it
If you forget to take a dose of quetiapine and you usually take it once a day, take the missed dose as soon as you remember, unless it's less than 12 hours until your next dose. In this case, skip the missed dose and take your next dose at the usual time.
If you forget a dose and you usually take it twice a day, take the missed dose as soon as you remember, unless it's less than 8 hours until your next dose. In this case, skip the missed dose and take your next dose at the usual time.
Do not take 2 doses at the same time to make up for a forgotten dose.
If you miss 2 or more doses, contact your doctor.
If you often forget doses it may help to set an alarm to remind you. You could also ask your pharmacist for advice on other ways to help you remember to take your medicine.
If you take too much
Taking too much quetiapine can cause serious side effects.
Urgent advice: Contact 111 for advice now if:
you take more than your prescribed dose of quetiapine and you:
- feel sleepy or tired
- feel dizzy
- have changes to your heartbeat
Immediate action required: Call 999 or go to A&E now if:
- you take more than your prescribed dose of quetiapine and you have a seizure or fit
If you need to go to A&E, do not drive yourself. Get someone else to drive you or call for an ambulance.
Take the quetiapine packet or leaflet inside it, plus any remaining medicine, with you.
Stopping quetiapine
Do not stop taking quetiapine unless your doctor tells you to, as your symptoms may come back.
If your doctor asks you to stop taking this medicine, your dose may be decreased slowly over a few days.
If you reduce your dose too quickly you may get withdrawal symptoms such as feeling or being sick (nausea or vomiting), sweating and problems sleeping.
Talk to your doctor if you have any problems when you reduce your dose or stop taking quetiapine.
0019
& nbsp,
Contraindications
with caution
Use during breastfeeding 9000
Tedu in the structure of bipolar disorder
Treatment of depressive episodes from moderate to severe severity in the structure of bipolar disorder
Disorders on the part of the nervous system:
Very often - dizziness 1. 4.17 , drowsiness 1.2.17 , headache 10 , extrapyramidal symptoms 1.13 ,
4.17 , drowsiness 1.2.17 , headache 10 , extrapyramidal symptoms 1.13 ,
66 Vascular disorders:
very common - increased blood pressure,
often - orthostatic hypotension 1,4,170029
Violations of the liver and biliary tract:
Infrequently - skin rash,
Metabolism and power disorders: 9000
Laboratory data:
1. & NBSP, & NBSP, & NBSP, & NBSP, & NBSP, & NBSP, & NBSP, & NBSP, & NBSP, & NBSP, & NBSP, & NBSP, & NBSP, & NBSP, & nbsp , , , , See Special Instructions section.
2. Drowsiness usually occurs within the first two weeks of starting therapy and usually resolves with continued use of quetiapine. nine0007
3. , , , , , , , Possible asymptomatic increase (≥ ,3 times the upper limit of normal when measured at any time) in plasma AST, ALT and GGT, as a rule, reversible with continued use of quetiapine.
4. , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , especially at the beginning of therapy (see section "Special Instructions"). nine0007
5. , , , , , , , Very rare cases of diabetes decompensation have been reported.
6. , , , , , , , The frequency of this side effect was estimated based on the results of a post-marketing study.
7. , , , , , , , Elevated fasting blood glucose ≥ ,126 ,mg/dl (≥ ,7.0 ,mmol/l) or postprandial blood glucose ≥ ,200 ,mg/dl (≥ ,11.1 ,mmol/l) at least once. nine0007
8. , , , , , , , , , , , , , , , , , , , , A higher incidence of dysphagia with quetiapine compared with placebo was noted only in patients with depression in the structure of bipolar disorder.
9. , , , , , , , Increase in initial body weight by at least 7 ,%. It mainly occurs at the beginning of therapy in adults.
10. , , , The frequency of the "withdrawal" syndrome was significantly reduced 1 week after discontinuation of quetiapine. nine0007
11. , , , Elevated triglycerides ≥ ,200 ,mg/dl ( ,2,258 ,mmol/l) in patients ≥ ,18 years or 150 ,mg/dl ( ,1,694 , mmol/l) in patients <, , 18 years old, at least with a single determination.
12. , , , Increase in total cholesterol ≥ ,240 ,mg/dl ( ,6,2064 ,mmol/l) in patients ≥ ,18 years or ≥ ,200 ,mg/dl (≥ ,5.172 ,mmol/l) in patients <, ,18 years of age at least once. nine0007
13. , , , See instructions below.
14. , , , Decrease in the number of platelets ≤ ,100 ,× ,10 9 /l at least with a single determination.
15. , , , Not associated with neuroleptic malignant syndrome. According to clinical studies.
16. , , , Increased prolactin concentration in patients ≥ ,18 years old: >, ,20 ,mcg/l (≥ ,869.56 & nbsp, pmol / l) in men, >, & nbsp, 30 & nbsp, μg / l (≥ & nbsp, 1304.34 pmol / l) in women.
17. , , , May cause falls.
18. , , , HDL cholesterol reduction <, ,40 ,mg/dl (<, ,1.03 ,mmol/l) in men and <, ,50 ,mg/dl (< , & nbsp, 1.29 & nbsp, mmol / l) in women.
19. , , , These phenomena were often noted against the background of tachycardia, dizziness, orthostatic hypotension and / or concomitant pathology of the cardiovascular or respiratory system. nine0007
20. , , , Based on potentially clinically significant abnormalities from baseline reported in all clinical studies. Changes in the concentration of total T 4 , free T 4 , total T 3 , free T 3 to <, ,80 ,% of the lower limit of normal (pmol / l) when measured at any time. Change in TSH concentration >, 5 mIU/L when measured at any time.
Change in TSH concentration >, 5 mIU/L when measured at any time.
21. , , , Based on increased incidence of vomiting in older patients (age ≥ ,65 years).
22. , , , , , , , , , ,10 9 /l cases of neutropenia (neutrophil count <, ,1 ,5 ,× ,10 9 /l) were observed in 1.9 ,% of patients in the quetiapine group versus 1.5 ,% in the placebo group. Reducing the number of neutrophils ≥ ,0.5 ,× ,10 9 /l, but <, ,1.0 ,× ,10 9 /l was observed with a frequency of 0.2 ,% in the quetiapine and placebo group. A decrease in the number of neutrophils <, ,0.5 ,× ,10 9 /l at least with a single determination was noted in 0.21 ,% of patients in the quetiapine group versus 0 ,% in the placebo group.
23. , , , A decrease in hemoglobin concentration ≤ ,13 ,g/dl in men and ≤ ,12 ,g/dl in women, at least with a single determination, was observed in 11 ,% of patients while taking quetiapine in all clinical trials, including long-term therapy. In short-term placebo-controlled studies, a decrease in hemoglobin concentration ≤ .13 g/dl in men and ≤ .12 g/dl in women, at least once determined, was observed in 8.3 .% of patients in the quetiapine group compared with 6 ,2 ,% in the placebo group. nine0007
In short-term placebo-controlled studies, a decrease in hemoglobin concentration ≤ .13 g/dl in men and ≤ .12 g/dl in women, at least once determined, was observed in 8.3 .% of patients in the quetiapine group compared with 6 ,2 ,% in the placebo group. nine0007
24. , , , Based on potentially clinically significant abnormalities from baseline observed in all clinical studies. Increase in the number of eosinophils ≥ .1 .× .10 9 /l when measured at any time.
25. , , , Based on potentially clinically significant abnormalities from baseline observed in all clinical studies. Reducing the number of leukocytes ≤ ,3 ,× ,10 9 /l when measured at any time.
26. , , , May develop during or after initiation of therapy and be accompanied by hypotension and/or syncope. The frequency is based on reports of bradycardia and associated adverse events in all clinical studies of quetiapine.
27. , , , Based on an estimate of the frequency in patients who participated in all clinical studies of quetiapine who experienced severe neutropenia (<, ,0.5 ,× ,10 9 /l) in combination with infections.
28. , , , See Pregnancy and Breastfeeding Use section.
29. , , , Concentration change from >, ,132 ,mmol/l to <, ,132 ,mmol/l at least once.
30. , , , Frequency of QTc interval change from <, ,450ms to ≥ ,450 ,ms with an increase of ≥ ,30 ,ms. In placebo-controlled studies, the number of patients who experienced a clinically significant increase in the QTc interval was similar in the quetiapine and placebo groups. nine0007
,
Prolongation of the QT interval, ventricular arrhythmia, sudden death, cardiac arrest, and bidirectional ventricular tachycardia are considered side effects inherent in antipsychotics.
The frequency of EPS in short-term clinical studies in adult patients with schizophrenia and mania in the structure of bipolar disorder was comparable in the quetiapine and placebo groups (patients with schizophrenia: 7. 8% in the quetiapine group and 8.0% in the placebo, mania group in the structure of bipolar disorder: 11.2% in the quetiapine group and 11.4% in the placebo group). nine0007
8% in the quetiapine group and 8.0% in the placebo, mania group in the structure of bipolar disorder: 11.2% in the quetiapine group and 11.4% in the placebo group). nine0007
The frequency of EPS in short-term clinical studies in adult patients with depression in the structure of bipolar disorder in the quetiapine group was 8.9%, in the placebo group - 3.8%,%. At the same time, the frequency of individual symptoms of EPS (such as akathisia, extrapyramidal disorders, tremor, dyskinesia, dystonia, anxiety, involuntary muscle contractions, psychomotor agitation and muscle rigidity) was usually low and did not exceed 4% in each of the therapeutic groups. In long-term clinical studies of quetiapine in schizophrenia and bipolar disorder in adults, the incidence of EPS was comparable in the quetiapine and placebo groups. nine0007
During therapy with quetiapine, there may be a dose-dependent decrease in the concentration of thyroid hormones. The frequency of potentially clinically significant changes in the concentration of thyroid hormones in short-term clinical studies for total T 4 was 3. 4% in the quetiapine group and 0.6% in the placebo group, for free T 4 - 0.7%,% in the quetiapine group vs. 0.1%,% in the placebo group, for total T 3 - 0.54 ,% in the quetiapine group vs. 0.0 ,% in the placebo group, for free T 3 - 0.2% in the quetiapine group versus 0.0% in the placebo group. The change in TSH concentration was noted with a frequency of 3.2% in the quetiapine group and 2.7% in the placebo group. In short-term clinical studies of monotherapy, the frequency of potentially clinically significant changes in T 3 and TSH was 0.0 ,% in the quetiapine and placebo group, for T 4 and TSH was 0.1 ,% in the quetiapine group versus 0.0 ,% in the placebo group. These changes are usually not associated with clinically significant hypothyroidism. The maximum decrease in total and free T 4 registered at the sixth week of quetiapine therapy, without further decrease in hormone levels during long-term treatment.
4% in the quetiapine group and 0.6% in the placebo group, for free T 4 - 0.7%,% in the quetiapine group vs. 0.1%,% in the placebo group, for total T 3 - 0.54 ,% in the quetiapine group vs. 0.0 ,% in the placebo group, for free T 3 - 0.2% in the quetiapine group versus 0.0% in the placebo group. The change in TSH concentration was noted with a frequency of 3.2% in the quetiapine group and 2.7% in the placebo group. In short-term clinical studies of monotherapy, the frequency of potentially clinically significant changes in T 3 and TSH was 0.0 ,% in the quetiapine and placebo group, for T 4 and TSH was 0.1 ,% in the quetiapine group versus 0.0 ,% in the placebo group. These changes are usually not associated with clinically significant hypothyroidism. The maximum decrease in total and free T 4 registered at the sixth week of quetiapine therapy, without further decrease in hormone levels during long-term treatment. In almost all cases, the concentration of total and free T 4 returned to baseline after discontinuation of quetiapine therapy, regardless of the duration of treatment. The concentration of thyroxin-binding globulin (TSG) when measured in 8 patients remained unchanged.
In almost all cases, the concentration of total and free T 4 returned to baseline after discontinuation of quetiapine therapy, regardless of the duration of treatment. The concentration of thyroxin-binding globulin (TSG) when measured in 8 patients remained unchanged.
,
Overdose Interaction with other medicinal products
Cytochrome P450 (CYP) 3A4 isoenzyme is the main isoenzyme responsible for the metabolism of quetiapine via the cytochrome P450 system. In healthy volunteers, the combined use of quetiapine (at a dose of 25 mg) with ketoconazole, an inhibitor of the CYP3A4 isoenzyme, led to an increase in the area under the concentration-time curve (AUC) of quetiapine by 5-8 times. nine0007
Warnings
Suicide/suicidal ideation or clinical deterioration
Depression in bipolar disorder is associated with an increased risk of suicidal ideation, self-harm and suicide (suicide-related events). This risk persists until a pronounced remission occurs. Due to the fact that it may take several weeks or more before the patient's condition improves from the start of treatment, patients should be under close medical supervision until improvement occurs. According to generally accepted clinical experience, the risk of suicide may increase in the early stages of remission. Patients (especially those at high risk of suicide) and their caregivers should be warned to monitor clinical deterioration, suicidal behavior or thoughts, unusual changes in behavior, and the need to immediately contact a doctor if they occur. According to clinical studies in patients with depression in bipolar disorder, the risk of suicide-related events was 3.0% (7/233) for quetiapine and 0% (0/120) for placebo in patients aged 18-24 years, 1.8% (19/1616) for quetiapine and 1.8% (11/622) for placebo in patients over 25 years of age.
Due to the fact that it may take several weeks or more before the patient's condition improves from the start of treatment, patients should be under close medical supervision until improvement occurs. According to generally accepted clinical experience, the risk of suicide may increase in the early stages of remission. Patients (especially those at high risk of suicide) and their caregivers should be warned to monitor clinical deterioration, suicidal behavior or thoughts, unusual changes in behavior, and the need to immediately contact a doctor if they occur. According to clinical studies in patients with depression in bipolar disorder, the risk of suicide-related events was 3.0% (7/233) for quetiapine and 0% (0/120) for placebo in patients aged 18-24 years, 1.8% (19/1616) for quetiapine and 1.8% (11/622) for placebo in patients over 25 years of age.
Other psychiatric disorders for which quetiapine is prescribed are also associated with an increased risk of suicidal events. In addition, such conditions may be comorbid with a depressive episode. Thus, precautions used in the treatment of patients with a depressive episode should also be taken in the treatment of patients with other psychiatric disorders.
Thus, precautions used in the treatment of patients with a depressive episode should also be taken in the treatment of patients with other psychiatric disorders.
Abrupt discontinuation of quetiapine therapy should take into account the potential risk of suicidal events. nine0007
Patients with a history of suicidal events, as well as patients who clearly express suicidal thoughts before starting therapy, are at increased risk of suicidal intentions and suicide attempts and should be carefully monitored during treatment. According to clinical studies in patients, suicide occurred in 3.0 & nbsp,% of cases of taking quetiapine versus 0 ,% of placebo in people under 25 years of age.
In children, adolescents and young adults (under 24 years of age) with depression, other mental disorders, antidepressants increase the risk of suicidal thoughts and suicidal behavior. nine0007
An FDA (Food and Drug Administration) meta-analysis of placebo-controlled trials of antidepressants, pooling data from approximately 4,400 children and adolescents and 7,700 adult patients with psychiatric disorders, found an increased risk of suicidal behavior with antidepressants compared with placebo in children, adolescents and adults under 25 years of age. This meta-analysis did not include studies using quetiapine (see Pharmacodynamics section). When prescribing quetiapine and any antidepressants in young people (at the age of 18-24 years), the risk of suicide should be correlated with the benefits of their use. nine0007
This meta-analysis did not include studies using quetiapine (see Pharmacodynamics section). When prescribing quetiapine and any antidepressants in young people (at the age of 18-24 years), the risk of suicide should be correlated with the benefits of their use. nine0007
,
Sleep apnea
Sleep apnea has been reported in patients taking quetiapine. Caution should be exercised when prescribing quetiapine to patients receiving drugs that have a depressant effect on the central nervous system, as well as to patients with risk factors for apnea (for example, overweight/obesity, male sex) or with a history of sleep apnea.
Drowsiness 9002
Dysphagia
Dysphagia (see side effects section) and aspiration have been observed with quetiapine therapy. A causal relationship between the occurrence of aspiration pneumonia and the use of quetiapine has not been established. However, caution should be exercised when prescribing the drug to patients at risk of aspiration pneumonia.
Constipation and bowel obstruction
Constipation is a risk factor for bowel obstruction. Against the background of the use of quetiapine, the development of constipation and intestinal obstruction was noted (see section "Side effects"), including cases with a fatal outcome in patients at high risk of intestinal obstruction, including those receiving multiple concomitant drugs that reduce intestinal motility, even in the absence of complaints for constipation. Patients with ileus/ileus should be closely monitored and in an emergency setting. nine0007
Pancreatitis
Pancreatitis has been reported during clinical trials, post-marketing studies and post-marketing use, but a causal relationship with the drug has not been established. Post-marketing reports indicated that many patients had risk factors for pancreatitis, such as elevated triglycerides, cholelithiasis, and alcohol use.
,
The impact on the ability to drive vehicles and mechanisms
Storage conditions
Shelf life
Vacation Conditions
Legal entity, in the name of the registration certificate 9000
Verthex, Russia
Legal address: 197350, St. Petersburg, Road to Kamenka, 62, lit. A.
Petersburg, Road to Kamenka, 62, lit. A.
,
Manufacturer
VERTEX JSC, Russia
Production address: St. Petersburg, Road to Kamenka, 62, lit. A.
Organization that accepts consumer claims:
VERTEKS JSC, Russia
199106, St. A.
Pharmacy on Maslennikova
,
,
,
6 Application during pregnancy and during breastfeeding
Method of application and dose
Treatment of schizophrenia
Treatment of manic episodes in the structure of bipolar disorder
Treatment of depressive episodes from the average grav bipolar disorder
Nervous system disorders:
very common - dizziness 1,4,17 , drowsiness 1,2,17 , headache 10 , extrapyramidal symptoms 1.13 ,
often - orthostatic hypotension 1,4,17 ,
Renal and urinary tract disorders:
Liver and biliary tract disorders:
infrequently 90.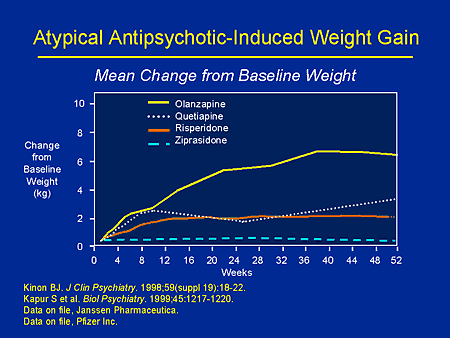 06 infrequently 90.060101
06 infrequently 90.060101 Metabolism and nutrition disorders:
Laboratory and instrumental data:
2. Drowsiness usually occurs within the first two weeks of starting therapy and usually resolves with continued use of quetiapine.
3. , , , , , , , Possible asymptomatic increase (≥ ,3 times the upper limit of normal when measured at any time) in plasma AST, ALT and GGT, as a rule, reversible with continued use of quetiapine. nine0007
4. , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , especially at the beginning of therapy (see section "Special Instructions").
5. , , , , , , , Very rare cases of diabetes decompensation have been noted. nine0007
6. , , , , , , , The frequency of this side effect was estimated based on the results of a post-marketing study.
7. , , , , , , , Elevated fasting blood glucose ≥ ,126 ,mg/dl (≥ ,7.0 ,mmol/l) or postprandial blood glucose ≥ ,200 ,mg/dl (≥ ,11.1 ,mmol/l) at least once. nine0007
8. , , , , , , , , , , , , , , , , , , , , A higher incidence of dysphagia with quetiapine compared with placebo was noted only in patients with depression in the structure of bipolar disorder.
9. , , , , , , , Increase in initial body weight by at least 7 ,%. It mainly occurs at the beginning of therapy in adults.
10. , , , The frequency of the "withdrawal" syndrome was significantly reduced 1 week after discontinuation of quetiapine. nine0007
11. , , , Elevated triglycerides ≥ ,200 ,mg/dl ( ,2,258 ,mmol/l) in patients ≥ ,18 years or 150 ,mg/dl ( ,1,694 , mmol/l) in patients <, , 18 years old, at least with a single determination.
12. , , , Increase in total cholesterol ≥ ,240 ,mg/dl ( ,6,2064 ,mmol/l) in patients ≥ ,18 years or ≥ ,200 ,mg/dl (≥ ,5.172 ,mmol/l) in patients <, ,18 years of age at least once. nine0007
13. , , , See instructions below.
14. , , , Decrease in the number of platelets ≤ ,100 ,× ,10 9 /l at least with a single determination.
15. , , , Not associated with neuroleptic malignant syndrome. According to clinical studies.
16. , , , Increased prolactin concentration in patients ≥ ,18 years old: >, ,20 ,mcg/l (≥ ,869.56 & nbsp, pmol / l) in men, >, & nbsp, 30 & nbsp, μg / l (≥ & nbsp, 1304.34 pmol / l) in women.
17. , , , May cause falls.
18. , , , HDL cholesterol reduction <, ,40 ,mg/dl (<, ,1.03 ,mmol/l) in men and <, ,50 ,mg/dl (< , & nbsp, 1. 29 & nbsp, mmol / l) in women.
29 & nbsp, mmol / l) in women.
19. , , , These phenomena were often noted against the background of tachycardia, dizziness, orthostatic hypotension and / or concomitant pathology of the cardiovascular or respiratory system. nine0007
20. , , , Based on potentially clinically significant abnormalities from baseline reported in all clinical studies. Changes in the concentration of total T 4 , free T 4 , total T 3 , free T 3 to <, ,80 ,% of the lower limit of normal (pmol / l) when measured at any time. Change in TSH concentration >, 5 mIU/L when measured at any time.
21. , , , Based on increased incidence of vomiting in older patients (age ≥ ,65 years).
22. , , , , , , , , , ,10 9 /l cases of neutropenia (neutrophil count <, ,1 ,5 ,× ,10 9 /l) were observed in 1.9 ,% of patients in the quetiapine group versus 1. 5 ,% in the placebo group. Reducing the number of neutrophils ≥ ,0.5 ,× ,10 9 /l, but <, ,1.0 ,× ,10 9 /l was observed with a frequency of 0.2 ,% in the quetiapine and placebo group. A decrease in the number of neutrophils <, ,0.5 ,× ,10 9 /l at least with a single determination was noted in 0.21 ,% of patients in the quetiapine group versus 0 ,% in the placebo group.
5 ,% in the placebo group. Reducing the number of neutrophils ≥ ,0.5 ,× ,10 9 /l, but <, ,1.0 ,× ,10 9 /l was observed with a frequency of 0.2 ,% in the quetiapine and placebo group. A decrease in the number of neutrophils <, ,0.5 ,× ,10 9 /l at least with a single determination was noted in 0.21 ,% of patients in the quetiapine group versus 0 ,% in the placebo group.
23. , , , A decrease in hemoglobin concentration ≤ ,13 ,g/dl in men and ≤ ,12 ,g/dl in women, at least with a single determination, was observed in 11 ,% of patients while taking quetiapine in all clinical trials, including long-term therapy. In short-term placebo-controlled studies, a decrease in hemoglobin concentration ≤ .13 g/dl in men and ≤ .12 g/dl in women, at least once determined, was observed in 8.3 .% of patients in the quetiapine group compared with 6 ,2 ,% in the placebo group. nine0007
nine0007
24. , , , Based on potentially clinically significant abnormalities from baseline observed in all clinical studies. Increase in the number of eosinophils ≥ .1 .× .10 9 /l when measured at any time.
25. , , , Based on potentially clinically significant abnormalities from baseline observed in all clinical studies. Reducing the number of leukocytes ≤ ,3 ,× ,10 9 /l when measured at any time.
26. , , , May develop during or after initiation of therapy and be accompanied by hypotension and/or syncope. The frequency is based on reports of bradycardia and associated adverse events in all clinical studies of quetiapine.
27. , , , Based on an estimate of the frequency in patients who participated in all clinical studies of quetiapine who experienced severe neutropenia (<, ,0.5 ,× ,10 9 /l) in combination with infections.
28. , , , See Pregnancy and Breastfeeding Use section.
, , , See Pregnancy and Breastfeeding Use section.
29. , , , Concentration change from >, ,132 ,mmol/l to <, ,132 ,mmol/l at least once.
30. , , , Frequency of QTc interval change from <, ,450ms to ≥ ,450 ,ms with an increase of ≥ ,30 ,ms. In placebo-controlled studies, the number of patients who experienced a clinically significant increase in the QTc interval was similar in the quetiapine and placebo groups. nine0007
,
Prolongation of the QT interval, ventricular arrhythmia, sudden death, cardiac arrest, and bidirectional ventricular tachycardia are considered side effects inherent in antipsychotics.
The frequency of EPS in short-term clinical studies in adult patients with schizophrenia and mania in the structure of bipolar disorder was comparable in the quetiapine and placebo groups (patients with schizophrenia: 7.8% in the quetiapine group and 8.0% in the placebo, mania group in the structure of bipolar disorder: 11. 2% in the quetiapine group and 11.4% in the placebo group). nine0007
2% in the quetiapine group and 11.4% in the placebo group). nine0007
The frequency of EPS in short-term clinical studies in adult patients with depression in the structure of bipolar disorder in the quetiapine group was 8.9%, in the placebo group - 3.8%,%. At the same time, the frequency of individual symptoms of EPS (such as akathisia, extrapyramidal disorders, tremor, dyskinesia, dystonia, anxiety, involuntary muscle contractions, psychomotor agitation and muscle rigidity) was usually low and did not exceed 4% in each of the therapeutic groups. In long-term clinical studies of quetiapine in schizophrenia and bipolar disorder in adults, the incidence of EPS was comparable in the quetiapine and placebo groups. nine0007
During therapy with quetiapine, there may be a dose-dependent decrease in the concentration of thyroid hormones. The frequency of potentially clinically significant changes in the concentration of thyroid hormones in short-term clinical studies for total T 4 was 3. 4% in the quetiapine group and 0.6% in the placebo group, for free T 4 - 0.7%,% in the quetiapine group vs. 0.1%,% in the placebo group, for total T 3 - 0.54 ,% in the quetiapine group vs. 0.0 ,% in the placebo group, for free T 3 - 0.2% in the quetiapine group versus 0.0% in the placebo group. The change in TSH concentration was noted with a frequency of 3.2% in the quetiapine group and 2.7% in the placebo group. In short-term clinical studies of monotherapy, the frequency of potentially clinically significant changes in T 3 and TSH was 0.0 ,% in the quetiapine and placebo group, for T 4 and TSH was 0.1 ,% in the quetiapine group versus 0.0 ,% in the placebo group. These changes are usually not associated with clinically significant hypothyroidism. The maximum decrease in total and free T 4 registered at the sixth week of quetiapine therapy, without further decrease in hormone levels during long-term treatment.
4% in the quetiapine group and 0.6% in the placebo group, for free T 4 - 0.7%,% in the quetiapine group vs. 0.1%,% in the placebo group, for total T 3 - 0.54 ,% in the quetiapine group vs. 0.0 ,% in the placebo group, for free T 3 - 0.2% in the quetiapine group versus 0.0% in the placebo group. The change in TSH concentration was noted with a frequency of 3.2% in the quetiapine group and 2.7% in the placebo group. In short-term clinical studies of monotherapy, the frequency of potentially clinically significant changes in T 3 and TSH was 0.0 ,% in the quetiapine and placebo group, for T 4 and TSH was 0.1 ,% in the quetiapine group versus 0.0 ,% in the placebo group. These changes are usually not associated with clinically significant hypothyroidism. The maximum decrease in total and free T 4 registered at the sixth week of quetiapine therapy, without further decrease in hormone levels during long-term treatment. In almost all cases, the concentration of total and free T 4 returned to baseline after discontinuation of quetiapine therapy, regardless of the duration of treatment. The concentration of thyroxin-binding globulin (TSG) when measured in 8 patients remained unchanged.
In almost all cases, the concentration of total and free T 4 returned to baseline after discontinuation of quetiapine therapy, regardless of the duration of treatment. The concentration of thyroxin-binding globulin (TSG) when measured in 8 patients remained unchanged.
,
Overdose Interaction with other medicinal products
Cytochrome P450 (CYP) 3A4 isoenzyme is the main isoenzyme responsible for the metabolism of quetiapine via the cytochrome P450 system. In healthy volunteers, the combined use of quetiapine (at a dose of 25 mg) with ketoconazole, an inhibitor of the CYP3A4 isoenzyme, led to an increase in the area under the concentration-time curve (AUC) of quetiapine by 5-8 times. nine0007
Warnings
Suicide/suicidal ideation or clinical deterioration
Depression in bipolar disorder is associated with an increased risk of suicidal ideation, self-harm and suicide (suicide-related events). This risk persists until a pronounced remission occurs. Due to the fact that it may take several weeks or more before the patient's condition improves from the start of treatment, patients should be under close medical supervision until improvement occurs. According to generally accepted clinical experience, the risk of suicide may increase in the early stages of remission. Patients (especially those at high risk of suicide) and their caregivers should be warned to monitor clinical deterioration, suicidal behavior or thoughts, unusual changes in behavior, and the need to immediately contact a doctor if they occur. According to clinical studies in patients with depression in bipolar disorder, the risk of suicide-related events was 3.0% (7/233) for quetiapine and 0% (0/120) for placebo in patients aged 18-24 years, 1.8% (19/1616) for quetiapine and 1.8% (11/622) for placebo in patients over 25 years of age.
Due to the fact that it may take several weeks or more before the patient's condition improves from the start of treatment, patients should be under close medical supervision until improvement occurs. According to generally accepted clinical experience, the risk of suicide may increase in the early stages of remission. Patients (especially those at high risk of suicide) and their caregivers should be warned to monitor clinical deterioration, suicidal behavior or thoughts, unusual changes in behavior, and the need to immediately contact a doctor if they occur. According to clinical studies in patients with depression in bipolar disorder, the risk of suicide-related events was 3.0% (7/233) for quetiapine and 0% (0/120) for placebo in patients aged 18-24 years, 1.8% (19/1616) for quetiapine and 1.8% (11/622) for placebo in patients over 25 years of age.
Other psychiatric disorders for which quetiapine is prescribed are also associated with an increased risk of suicidal events. In addition, such conditions may be comorbid with a depressive episode. Thus, precautions used in the treatment of patients with a depressive episode should also be taken in the treatment of patients with other psychiatric disorders.
Thus, precautions used in the treatment of patients with a depressive episode should also be taken in the treatment of patients with other psychiatric disorders.
Abrupt discontinuation of quetiapine therapy should take into account the potential risk of suicidal events. nine0007
Patients with a history of suicidal events, as well as patients who clearly express suicidal thoughts before starting therapy, are at increased risk of suicidal intentions and suicide attempts and should be carefully monitored during treatment. According to clinical studies in patients, suicide occurred in 3.0 & nbsp,% of cases of taking quetiapine versus 0 ,% of placebo in people under 25 years of age.
In children, adolescents and young adults (under 24 years of age) with depression, other mental disorders, antidepressants increase the risk of suicidal thoughts and suicidal behavior. nine0007
An FDA (Food and Drug Administration) meta-analysis of placebo-controlled trials of antidepressants, pooling data from approximately 4,400 children and adolescents and 7,700 adult patients with psychiatric disorders, found an increased risk of suicidal behavior with antidepressants compared with placebo in children, adolescents and adults under 25 years of age.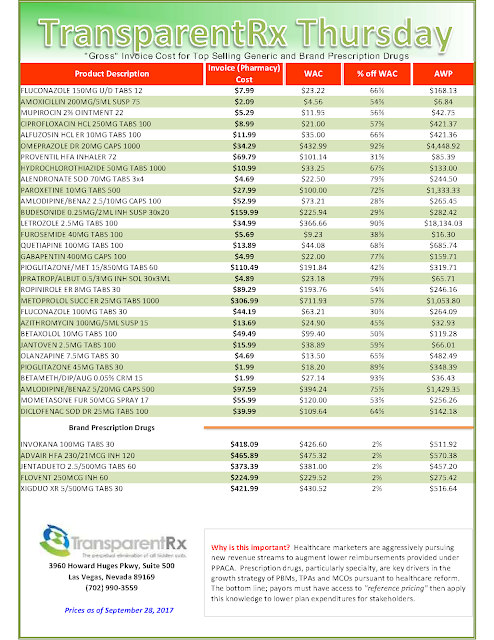 This meta-analysis did not include studies using quetiapine (see Pharmacodynamics section). When prescribing quetiapine and any antidepressants in young people (at the age of 18-24 years), the risk of suicide should be correlated with the benefits of their use. nine0007
This meta-analysis did not include studies using quetiapine (see Pharmacodynamics section). When prescribing quetiapine and any antidepressants in young people (at the age of 18-24 years), the risk of suicide should be correlated with the benefits of their use. nine0007
,
Sleep apnea
Sleep apnea has been reported in patients taking quetiapine. Caution should be exercised when prescribing quetiapine to patients receiving drugs that have a depressant effect on the central nervous system, as well as to patients with risk factors for apnea (for example, overweight/obesity, male sex) or with a history of sleep apnea.
Drowsiness 9002
Dysphagia
Dysphagia (see side effects section) and aspiration have been observed with quetiapine therapy. A causal relationship between the occurrence of aspiration pneumonia and the use of quetiapine has not been established. However, caution should be exercised when prescribing the drug to patients at risk of aspiration pneumonia.
Constipation and bowel obstruction
Constipation is a risk factor for bowel obstruction. Against the background of the use of quetiapine, the development of constipation and intestinal obstruction was noted (see section "Side effects"), including cases with a fatal outcome in patients at high risk of intestinal obstruction, including those receiving multiple concomitant drugs that reduce intestinal motility, even in the absence of complaints for constipation. Patients with ileus/ileus should be closely monitored and in an emergency setting. nine0007
Pancreatitis
Pancreatitis has been reported during clinical trials, post-marketing studies and post-marketing use, but a causal relationship with the drug has not been established. Post-marketing reports indicated that many patients had risk factors for pancreatitis, such as elevated triglycerides, cholelithiasis, and alcohol use.
,
The impact on the ability to drive vehicles and mechanisms
Storage conditions
Shelf life
Vacation Conditions
Legal entity, in the name of the registration certificate 9000
Verthex, Russia
Legal address: 197350, St.