Muscular dystrophy vs multiple sclerosis
Association of Limb-Girdle muscular dystrophy with multiple sclerosis: A case report
Caspian J Intern Med. 2018 Winter; 9(1): 96–99.
doi: 10.22088/cjim.9.1.96
, MD,1, MD,2 and , MD1,3,*
Author information Article notes Copyright and License information Disclaimer
Background:
The association of limb-girdle muscular dystrophy (LGMD) with other neurological disorders is uncommon.
Case presentation:
We report a 25-year-old female with LGMD who suffered from slowly progressive proximal muscular weakness and atrophy since she was 12 years of age. The patient recently presented with acute loss of left side visual acuity. After evaluation, findings were suggestive of multiple sclerosis.
Conclusions:
This is the first report of LGMD in association with MS. The simultaneous occurrence of MS with myopathies may be incidental but there may be a genetic susceptibility for both diseases. This comorbidity may influence the treatment of MS.
Key Words: Limb-Girdle Muscular Dystrophy, Multiple Sclerosis, Myopathy
Limb-girdle muscular dystrophy (LGMD) denominates a group of diseases that involve proximal muscles in childhood or adulthood. It is sometimes associated with elevated creatine phosphokinase serum levels and indicated by dystrophic changes (regeneration/degeneration) on muscle biopsy. The age of onset, severity, and systemic organ involvement varies among different subtypes. In addition, the signs and symptoms are different among the members of one family (1, 2). We report a known case of LGMD that presented with left optic neuritis during the follow-up. After investigation, a diagnosis of multiple sclerosis (MS) was made based on the magnetic resonance imaging (MRI) findings.
The patient was a 25-year-old female born to non-consanguineous parents. She was the second child of a family with one brother and two sisters. Her initial symptom began when she was 12 years old and presented as difficulty in climbing stairs. Her weakness was slowly progressive with the wasting of the proximal leg muscles so that she lost her ability to run. After a few years, she also felt weak in her shoulders and arms but to a lesser degree than her legs. She did not have any sensory or sphincter complaints. Her swallowing was normal. A family history of muscle weakness was positive in her grandmother, but it was not debilitating enough to look for a cause. One of her aunts had MS. Her history was negative for alcohol and smoking. She only took multivitamin pills temporarily. On examination, her body-mass index (BMI) was 23 and she was afebrile with no abnormal findings on physical examination of the skin, mucous, head, and neck. Cardiopulmonary and abdominopelvic examinations showed no abnormalities. She was fully obedient and her mental functions were intact with normal speech. An examination of her cranial nerves showed no facial or bulbar muscle involvement. Muscle weakness and atrophy was symmetrical, prominent in the pelvic and shoulder girdle sparing oculofaciobulbar muscles.
Her weakness was slowly progressive with the wasting of the proximal leg muscles so that she lost her ability to run. After a few years, she also felt weak in her shoulders and arms but to a lesser degree than her legs. She did not have any sensory or sphincter complaints. Her swallowing was normal. A family history of muscle weakness was positive in her grandmother, but it was not debilitating enough to look for a cause. One of her aunts had MS. Her history was negative for alcohol and smoking. She only took multivitamin pills temporarily. On examination, her body-mass index (BMI) was 23 and she was afebrile with no abnormal findings on physical examination of the skin, mucous, head, and neck. Cardiopulmonary and abdominopelvic examinations showed no abnormalities. She was fully obedient and her mental functions were intact with normal speech. An examination of her cranial nerves showed no facial or bulbar muscle involvement. Muscle weakness and atrophy was symmetrical, prominent in the pelvic and shoulder girdle sparing oculofaciobulbar muscles.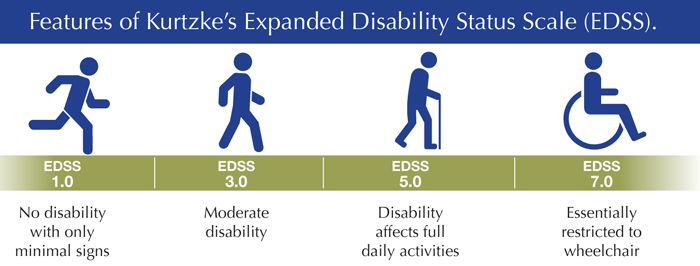
The muscles had a normal tonus without any evidence of contracture or calf hypertrophy. She had good strength in her axial muscles (5/5). Weakness was more obvious in the lower limbs than in the upper limbs (3/5 and 4/5, respectively). The power of her distal muscles, wrists, and feet was preserved. Her tendon reflexes diminished proportional to their weakness. Plantar reflexes were down bilaterally. Sensory examination was normal. Laboratory tests performed at that time showed an increased level of transaminases (ALT: 72 U/L, AST: 55U/L) and CPK 10000 U/L. Cardiologic consultation revealed no significant cardiac abnormalities.
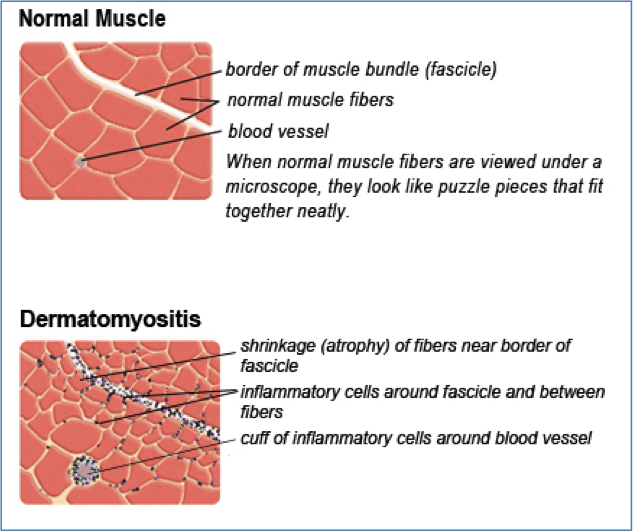
The first electromyography (EMG) (2005) showed a myopathic pattern in the lower limbs from which a muscle biopsy was requested but the patient postponed it. Until the patient was 20 years old, her symptoms were progressive; then, she experienced a stabilization of the disease. A biopsy taken in 2011 revealed dystrophic changes (degeneration/ regeneration) with markedly increased endomysial connective tissue; no inflammatory cell infiltration, ragged red fiber, cytoplasmic body and lipid or glycogen accumulation in muscle fibers were seen ().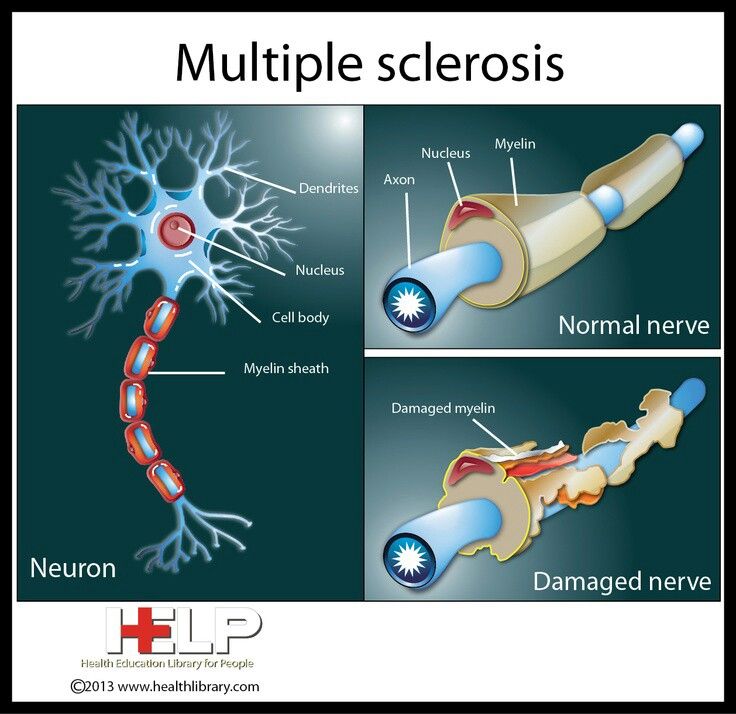 An immunohistochemistry (IHC) study of sarcolemmal proteins showed sarcolemma labeling of all fibers with DYS1, DYS2, DYS3, alpha and gamma sarcoglycan, dysferlin, merosin, and beta-spectrin ().
An immunohistochemistry (IHC) study of sarcolemmal proteins showed sarcolemma labeling of all fibers with DYS1, DYS2, DYS3, alpha and gamma sarcoglycan, dysferlin, merosin, and beta-spectrin ().
Open in a separate window
H&E staining: atrophy, some necrotic and regenerative fibers are noted. Endomysial connective tissue is severely increased
Open in a separate window
Immunohistochemistry (IHC) shows staining with anti-dystrophin, dysferlin, merosin, and sarcoglycan
The second EMG in 2011 showed a myopathic process pronounced in the proximal muscles. With a diagnosis of LGMD, rehabilitation, physical therapy, and stretching exercises were advised to improve her mobility and prevent disused atrophy or contracture deformities. In April 2015, she complained of acute left visual loss for four days with painful eye movements that lasted for several days. Her muscle strength had not changed for 5 years. On examination of her pupils, the left one had 2+ relative afferent pupillary defects (RAPD).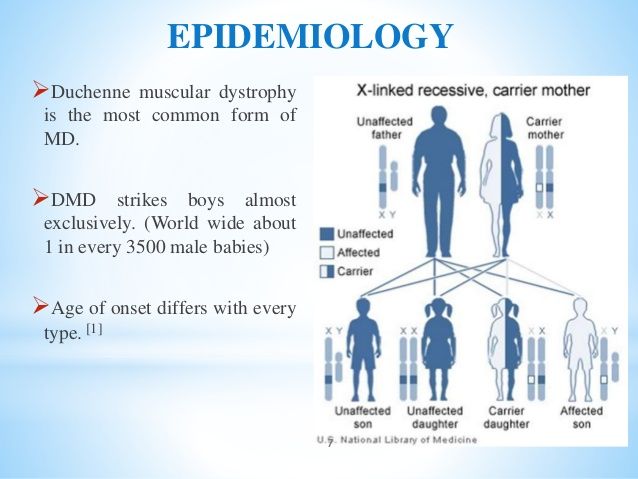 Left visual acuity was finger counting from one meter with cecocentral scotoma in the visual field. Extraocular muscles and other cranial nerves were intact. Ophthalmologic consultation revealed no bulbar ophthalmic problems. For further evaluation, we planned a brain and optic pathway MRI that excluded compressive lesions of the optic pathway and disclosed multiple periventricular, centrum semiovale, corpus callosum, juxta cortical, and infra tentorial (brain stem and cerebellum) T2 hypersignal demyelinating plaques with multiple lesion enhancement () that fulfilled the McDonald criteria for MS (3, 4).
Left visual acuity was finger counting from one meter with cecocentral scotoma in the visual field. Extraocular muscles and other cranial nerves were intact. Ophthalmologic consultation revealed no bulbar ophthalmic problems. For further evaluation, we planned a brain and optic pathway MRI that excluded compressive lesions of the optic pathway and disclosed multiple periventricular, centrum semiovale, corpus callosum, juxta cortical, and infra tentorial (brain stem and cerebellum) T2 hypersignal demyelinating plaques with multiple lesion enhancement () that fulfilled the McDonald criteria for MS (3, 4).
Open in a separate window
a) Sagittal T2 MRI, multiple hyperintense lesions involving periventricular white matter thalamus and cerebellum; b) Sagittal T1-weighted MRI showed enhancement of one periventricular lesion; c) and d) Axial T2 and FLAIR MRI, respectively; demonstrating multiple periventricular, centrum semioval, and juxtacortical lesions
From this, we prescribed intravenous methyl prednisolone (5 gr divided in 5 days) for the treatment of her optic neuritis. She responded significantly and her visual acuity ameliorated so that she could read from 30 cm. Vasculitis tests and anti-aquaporin 4 antibodies were negative. The final diagnosis was relapsing-remitting MS (RRMS). For long term treatment, we decided to put her on a glatiramer acetate daily injection.
She responded significantly and her visual acuity ameliorated so that she could read from 30 cm. Vasculitis tests and anti-aquaporin 4 antibodies were negative. The final diagnosis was relapsing-remitting MS (RRMS). For long term treatment, we decided to put her on a glatiramer acetate daily injection.
This is the first report of LGMD in association with MS. Mutations in more than 50 loci have been known for LGMD. LGMD is distinct from x-linked muscular dystrophy by the autosomal pattern of inheritance, and from facioscapulohumeral and Emery muscular dystrophy by sparing the facial muscles. A proximal muscle involvement will become evident either in late childhood or early adulthood and is relatively benign in comparison with Duchenne dystrophy. Cardiac involvement occurs in some subtypes of the disease and mental functions are usually normal (5-8).
No definitive treatment exists for LGMD; however, management should be based for weight control, rehabilitation, physiotherapy, orthopedic surgery for skeletal deformities, and if respiratory failure occurs, then the use of respiratory aids and a mechanical ventilator is required (9-11).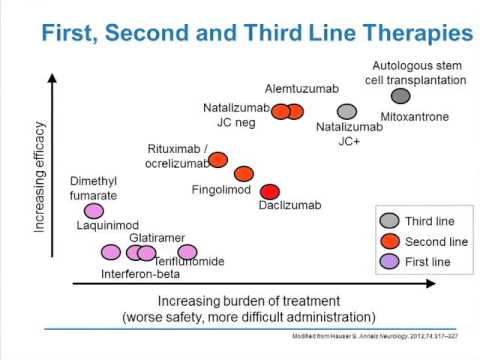 Although some reports suggest an association between myopathy and other neurological disorders, evidence is lacking to conclude this relationship.
Although some reports suggest an association between myopathy and other neurological disorders, evidence is lacking to conclude this relationship.
MS is another disabling neurologic disease, the most common autoimmune disorder affecting the central nervous system and is believed to occur as a result of genetics (including HLA DR15 and DQ56) and environmental factors (12). In recent years, the role of mitochondrial dysfunction in axonal loss degeneration in the progressive stage of MS has been investigated (13).
Some cases of MS and myopathy have been reported, for example, one case with centronuclear myopathy and RRMS (14). A 31-year-old woman with myotonic dystrophy type 2 presented with left hemihyperesthesia and mild spinal cord and brain stem symptoms whose paraclinical findings provided a diagnosis of RRMS (15). Two cases with mitochondrial myopathy and MS have been reported (16). The association of facioscapulohumeral muscular dystrophy and MS in a man with a history of bilateral pectoralis, proximal weakness, and atrophy of upper extremities since he was 16 years old showed left optic neuritis as the first presentation of MS at age 23 (17).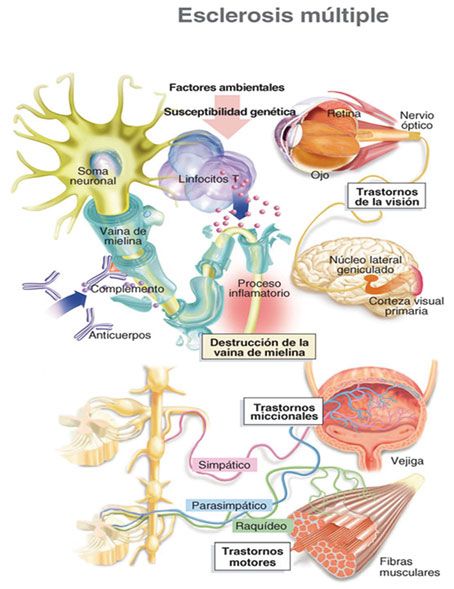 A 27-year-old woman with progressive proximal lower limb weakness and increased liver function tests since her 20s with evidence of MS based on a brain MRI, cerebrospinal fluid, and visual evoked potential and in combination with an observation of periodic acid-Schiff (PAS) positive vacuoles on her muscle biopsy that was conclusive for adult onset Pompe disease with PPMS (18). The simultaneous occurrence of MS with myopathies may be incidental but there may be a genetic susceptibility for both diseases.
A 27-year-old woman with progressive proximal lower limb weakness and increased liver function tests since her 20s with evidence of MS based on a brain MRI, cerebrospinal fluid, and visual evoked potential and in combination with an observation of periodic acid-Schiff (PAS) positive vacuoles on her muscle biopsy that was conclusive for adult onset Pompe disease with PPMS (18). The simultaneous occurrence of MS with myopathies may be incidental but there may be a genetic susceptibility for both diseases.
In conclusion the comorbidity between MS and others medical disorders is important for the treatment of this autoimmune disease. As the authors know this is the first report of MS in association with LGMD.
We thank the MS Research Center of Sina Hospital for their support.
The authors declare no conflict of interest.
1. Norwood F, de Visser M, Eymard B, et al. EFNS guideline on diagnosis and management of limb girdle muscular dystrophies. Eur J Neurol. 2007;14:1305–12. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
2. Beckmann J, Fardeau M. Limb-girdle muscular dystrophies. In: Emery A, editor. Neuromuscular disorders:clinical and molecular genetics. 1st ed. Chichester: John Wiley & Sons; 1998. pp. 123–56. [Google Scholar]
3. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. [PMC free article] [PubMed] [Google Scholar]
4. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol. 2005;58:840–6. [PubMed] [Google Scholar]
5. de Morree A, Hensbergen PJ, van Haagen HH, et al. Proteomic analysis of the dysferlin protein complex unveils its importance for sarcolemmal maintenance and integrity. PloS One. 2010;5:e13854. [PMC free article] [PubMed] [Google Scholar]
6. Panegyres PK, Mastaglia FL, Kakulas BA. Limb girdle syndromes. Clinical, morphological and electrophysiological studies. J Neurol Sci. 1990;95:201–18. [PubMed] [Google Scholar]
J Neurol Sci. 1990;95:201–18. [PubMed] [Google Scholar]
7. Pramono ZA, Lai PS, Tan CL, Takeda S, Yee WC. Identification and characterization of a novel human dysferlin transcript: dysferlin_v1. Hum Genet. 2006;120:410–9. [PubMed] [Google Scholar]
8. Pramono ZA, Tan , CL , Seah IA, et al. Identification and characterisation of human dysferlin transcript variants: implications for dysferlin mutational screening and isoforms. Human Genet. 2009;125:413–20. [PubMed] [Google Scholar]
9. Narayanaswami P, Carter G, David W, Weiss M, Amato AA. Evidence-based guideline summary: diagnosis and treatment of limb-girdle and distal dystrophies: report of the guideline development subcommittee of the american academy of neurology and the practice issues review panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology. 2015;84:1720–1. [PubMed] [Google Scholar]
10. Narayanaswami P, Weiss M, Selcen D, et al. Evidence-based guideline summary: diagnosis and treatment of limb-girdle and distal dystrophies: report of the guideline development subcommittee of the American academy of neurology and the practice issues review panel of the American Association of neuromuscular & electrodiagnostic medicine. Neurology. 2014;83:1453–63. [PMC free article] [PubMed] [Google Scholar]
Neurology. 2014;83:1453–63. [PMC free article] [PubMed] [Google Scholar]
11. Topaloglu H. Evidence-based guideline summary: Diagnosis and treatment of limb-girdle and distal dystrophies: Report of the guideline development subcommittee of the American academy of neurology and the practice issues review panel of the american association of neuromuscular & electrodiagnostic medicine. Neurology. 2015;84:1720. [PubMed] [Google Scholar]
12. Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. [PubMed] [Google Scholar]
13. Andrews HE NP, Bates D, Turnbull DM. Mitochondrial dysfunction plays a key role in progressive axonal loss in multiple sclerosis. Med Hypotheses. 2005;64:669–77. [PubMed] [Google Scholar]
14. Olsen DB, Langkilde AR, Schmalbruch H, Vissing J. Diagnostic challenges in combined multiple sclerosis and centronuclear myopathy. Eur J Neurol. 2000;7:567–71. [PubMed] [Google Scholar]
15. Terrence CF. Myotonic dystrophy and multiple sclerosis.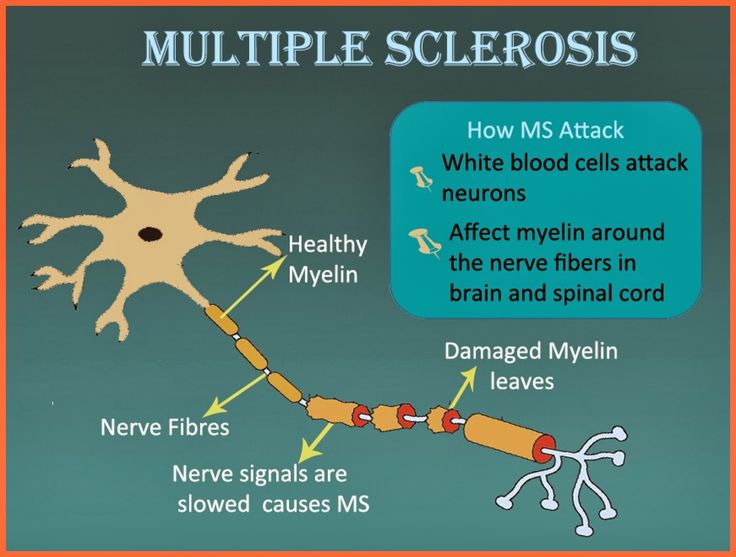 J Neurol. 1976;213:305–8. [PubMed] [Google Scholar]
J Neurol. 1976;213:305–8. [PubMed] [Google Scholar]
16. Bet L, Moggio M, Comi GP, et al. Multiple sclerosis and mitochondrial myopathy: an unusual combination of disease. J Neurol. 1994;241:511–6. [PubMed] [Google Scholar]
17. Mishra SK, Currier RD, Smith EE, et al. Facioscapulohumeral dystrophy associated with multiple sclerosis. Arch Neurol. 1984;41:570–1. [PubMed] [Google Scholar]
18. Sepulveda M, Munteis E, Rubio MA, Pascual J, Roquer J. Adult onset Pompe disease associated with multiple sclerosis. J Neurol. 2011;258:2286–7. [PubMed] [Google Scholar]
What is the Difference Between Multiple Sclerosis (MS) and Muscular Dystrophy (MD)?
`;
Health
Fact Checked
There are many differences between multiple sclerosis (MS) and muscular dystrophy (MD).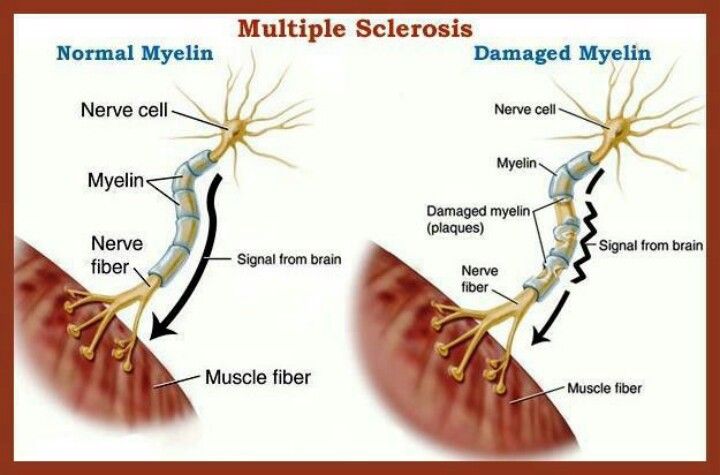 MS is believed to be an autoimmune disease that affects the nervous system, while MD is a group of related conditions that affect the muscles. People often confuse the two because their initials are so close, and the diseases may have similar symptoms in some cases.
MS is believed to be an autoimmune disease that affects the nervous system, while MD is a group of related conditions that affect the muscles. People often confuse the two because their initials are so close, and the diseases may have similar symptoms in some cases.
Causes
In multiple sclerosis, the body's immune system appears to attack the outer layer of the nerves, particularly in the brain, spinal cord, and optic nerve, eventually breaking this layer (called myelin) down. This means that signals sent along those nerves are slowed down or cannot travel at all. No one is sure why this happens, so researchers don't know the exact causes of the disease. It is thought that genetics likely plays a part, but other factors — like a virus or environmental conditions — may be involved.
This means that signals sent along those nerves are slowed down or cannot travel at all. No one is sure why this happens, so researchers don't know the exact causes of the disease. It is thought that genetics likely plays a part, but other factors — like a virus or environmental conditions — may be involved.
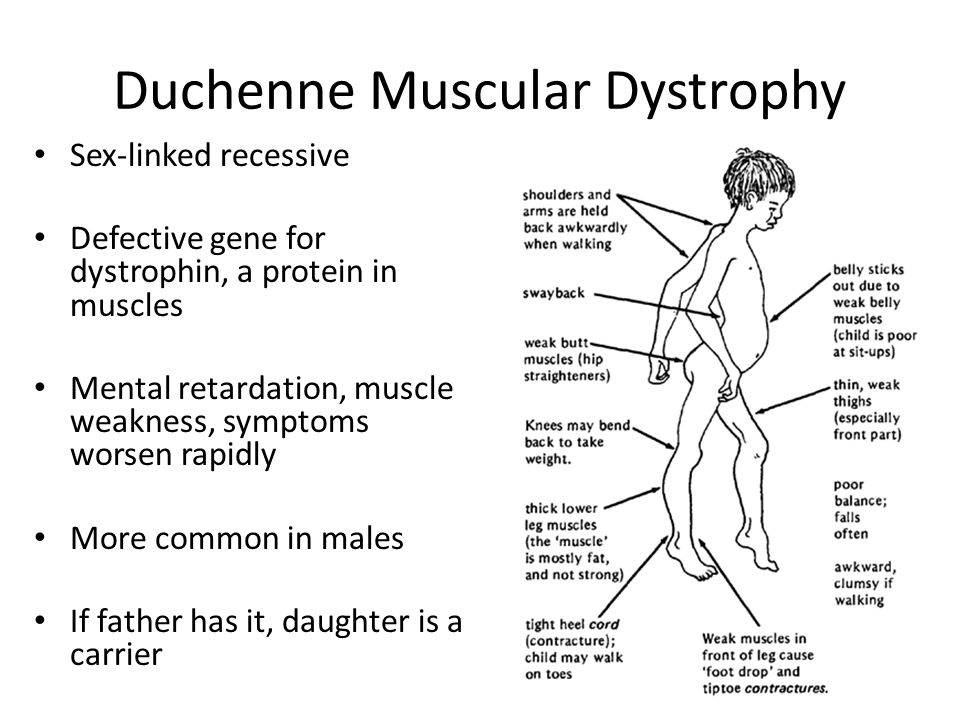
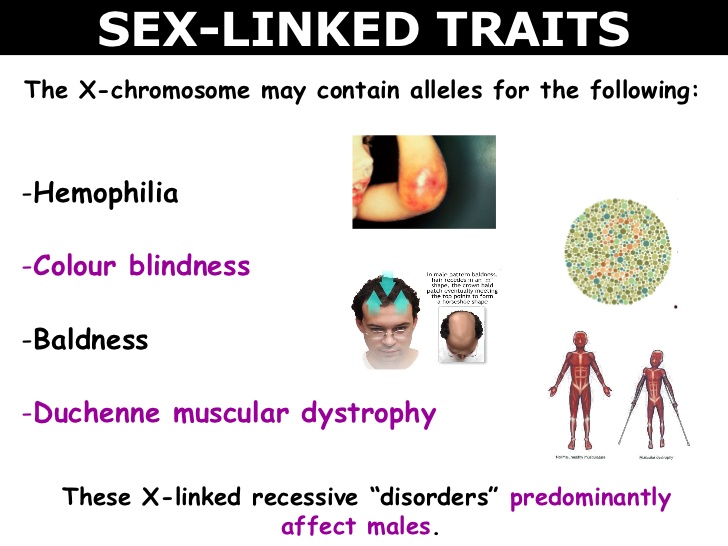
Muscular dystrophy, on the other hand, is genetic. In most cases, a gene that controls the production of certain types of proteins doesn't work correctly, causing muscle fibers to break down. There are many different types of MD, including a number of other closely related conditions, so the exact causes vary with the type of MD.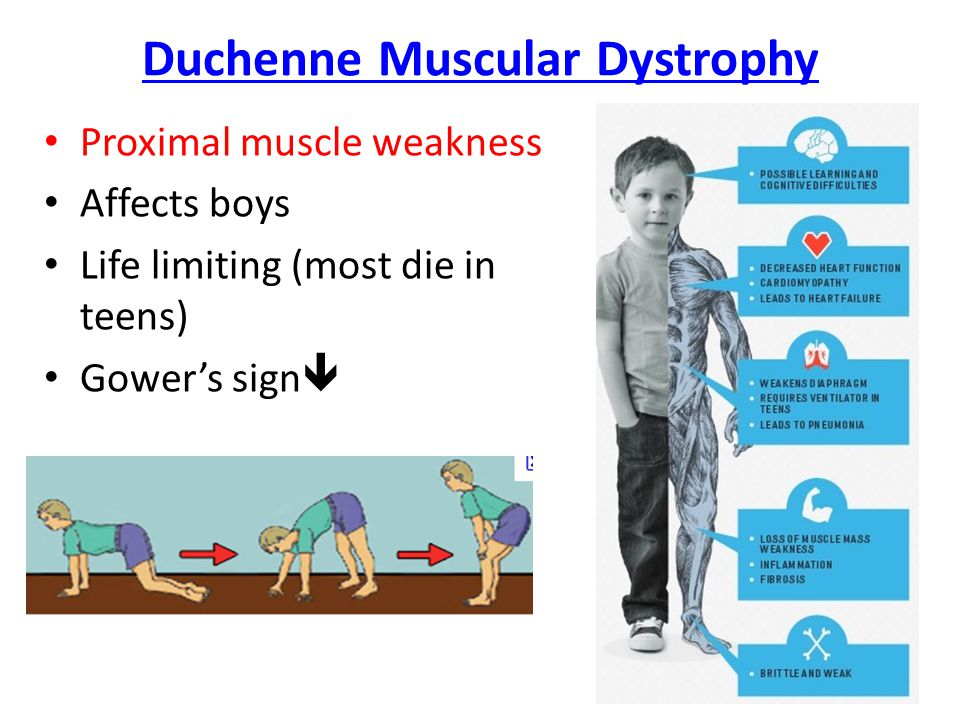 Duchenne MD is believed to be one of the most common forms. It's caused by a defective gene on the X chromosome and prevents the body from producing a protein called dystrophin, which is needed for the cell membranes of muscle fibers.
Duchenne MD is believed to be one of the most common forms. It's caused by a defective gene on the X chromosome and prevents the body from producing a protein called dystrophin, which is needed for the cell membranes of muscle fibers.
Who Is Affected?
MS is more common in women than men, which is also true of other autoimmune diseases. Diagnosis typically occurs between the ages of 20 and 50, although it can affect younger people as well. How genetics plays a role in MS isn't clear, but people who have family members with the disease are much more likely to develop it as well. It affects people of nearly all races, although people of northern European ancestry seem more susceptible, as are people who live farther from the equator in general.
How genetics plays a role in MS isn't clear, but people who have family members with the disease are much more likely to develop it as well. It affects people of nearly all races, although people of northern European ancestry seem more susceptible, as are people who live farther from the equator in general.
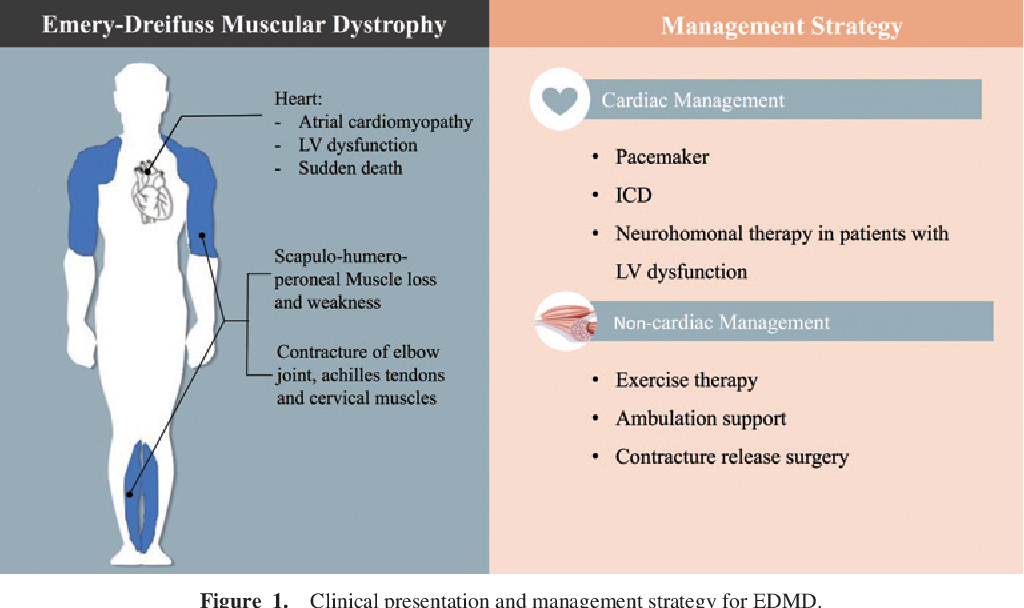
The most common types of muscular dystrophy affect boys, often those who are very young. The symptoms of Duchenne MD often start before the age of 5, and it's usually well advanced by the time a boy is 12. A similar condition, Becker muscular dystrophy, often develops when a child is older, often around age 10 or even later.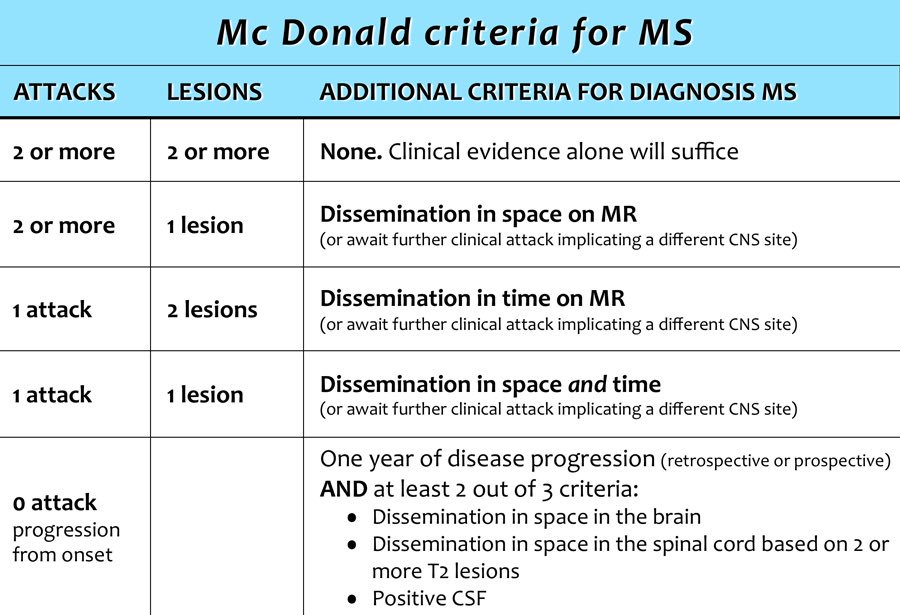 The symptoms of Emery-Dreifuss MD are usually present by the age of 10, and heart problems often develop by 20.
The symptoms of Emery-Dreifuss MD are usually present by the age of 10, and heart problems often develop by 20.
Other types of muscular dystrophy, including limb-girdle, myotonic, fascioscapulohumeral (FSHD), and congenital MD, can affect both men and women. There are congenital forms of MD that are present at the time of birth, whereas other types often develop when a person is in his or her teens or 20s. Oculopharyngeal MD usually does not appear until later in life, often after a person is in his or her 50s.
Symptoms
Some of the symptoms of multiple sclerosis and muscular dystrophy can be similar, which is one reason why they may be confused. Muscle weakness is common in both conditions, and problems walking and running can be seen in MS and some types of MD. People with MS are more likely to develop additional symptoms, like dizziness, vision problems, tingling or numbness, and feelings of electric shocks. Different forms of MD affect different parts of the body, so muscle weakness may be seen mostly in one area: the face with myotonic MD, for example, or the shoulders with FSHD.
Muscle weakness is common in both conditions, and problems walking and running can be seen in MS and some types of MD. People with MS are more likely to develop additional symptoms, like dizziness, vision problems, tingling or numbness, and feelings of electric shocks. Different forms of MD affect different parts of the body, so muscle weakness may be seen mostly in one area: the face with myotonic MD, for example, or the shoulders with FSHD.
MD tends to be very difficult on the body as the muscle fibers break down. Most forms will ultimately cause death as the muscles become weak and atrophied.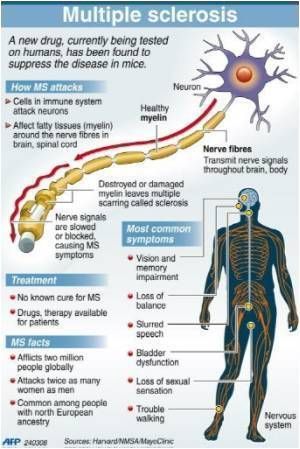 People with some of the most common forms often are unable to walk as the disease progresses, and may develop problems with their joints and spinal cord.
People with some of the most common forms often are unable to walk as the disease progresses, and may develop problems with their joints and spinal cord.
Multiple sclerosis affects the central nervous system, which can then result in movement difficulties. In this condition, the muscles may become harder to move without pain, and can atrophy because they're not being used. Muscle spasms and coordination problems can also occur. Symptoms often can be triggered or worsened by exposure to heat.
Those with MS usually don't have constant symptoms, although an attack can last for months in some cases.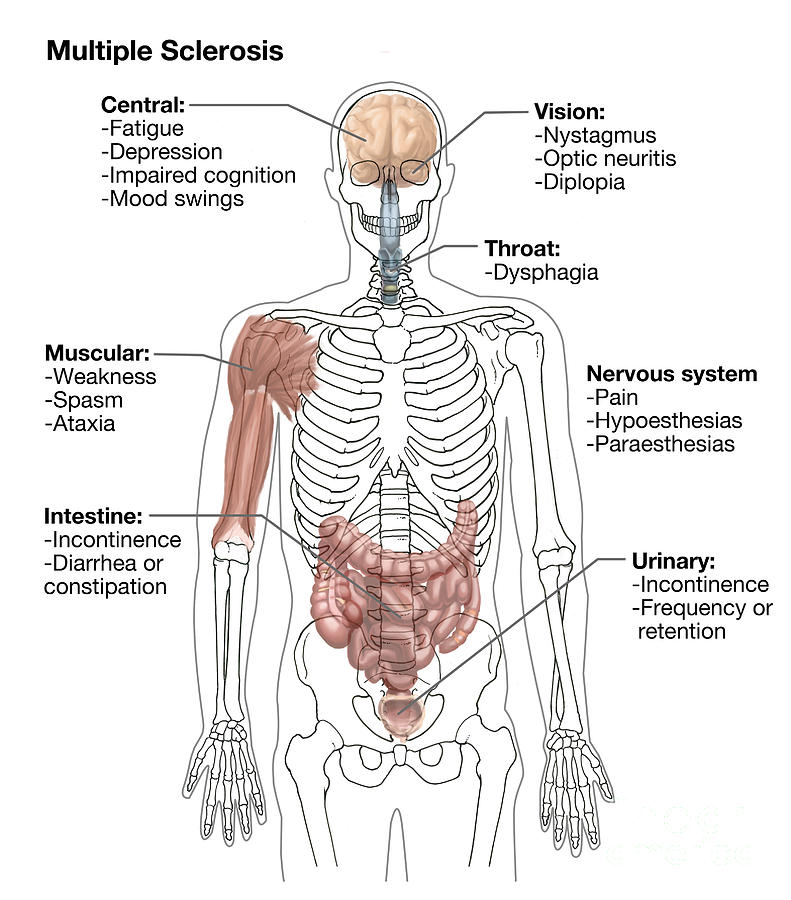 These attacks can temporarily impair movement, cause vision problems, and give the sufferer great pain. Symptoms often go away, especially in the early stages of the disease, and a person with the condition may go through long periods with no problems at all. As the disease worsens, however, attacks may become more frequent, and the person less likely to recover from them.
These attacks can temporarily impair movement, cause vision problems, and give the sufferer great pain. Symptoms often go away, especially in the early stages of the disease, and a person with the condition may go through long periods with no problems at all. As the disease worsens, however, attacks may become more frequent, and the person less likely to recover from them.
Diagnosis
A medical professional typically diagnoses MS based on MRI scans of the brain and spinal cord, nerve function tests, and a lumbar puncture. He or she will also typically examine the eyes for irregular responses of the pupils and other vision problems. A neurological exam to test movement in the arms and legs, reflexes, and changes in sensation in any part of the body may also be conducted.
He or she will also typically examine the eyes for irregular responses of the pupils and other vision problems. A neurological exam to test movement in the arms and legs, reflexes, and changes in sensation in any part of the body may also be conducted.
Tests for MD include muscle function tests and a muscle biopsy in some cases. Some types often have clear physical symptoms. Blood tests are often performed to find the levels of certain enzymes, including creatine kinase. DNA tests may be used to look for specific genetic mutations found in some forms of the disease.
Treatments
Currently, there is no known cure for either multiple sclerosis or muscular dystrophy.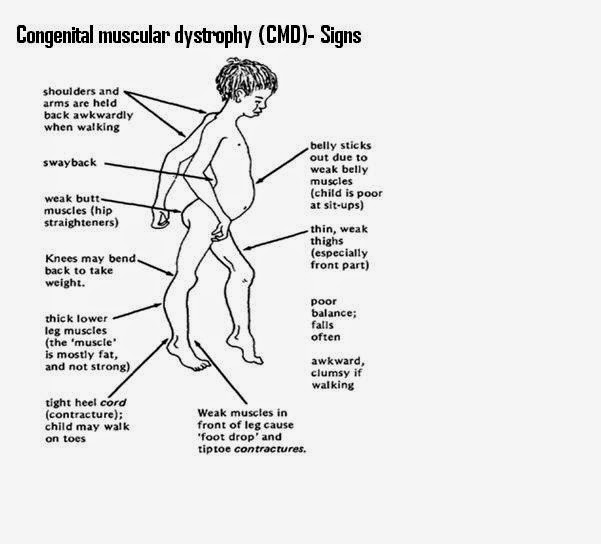 For both conditions, treatment is often focused on managing the symptoms and helping the patient maintain a good quality of life while living with the condition.
For both conditions, treatment is often focused on managing the symptoms and helping the patient maintain a good quality of life while living with the condition.
Some medications, including fingolimod and interferons, may help reduce the number of MS attacks and slow progression of the disease in some patients. Corticosteroids are often used to relieve the inflammation associated with attacks, and muscle relaxants may help with the pain and stiffness. Physical therapy is often recommended to help the patient maintain muscle tone.
People with MD may also experience benefits from physical therapy, which often helps the person keep more muscle function. As with MS, corticosteroids are often prescribed, and may help maintain muscle strength. Once the disease progresses, wheelchairs and braces may be needed so that the patient can stay mobile. People with spinal problems related to MD may need surgery to make breathing easier. A pacemaker may also be required if the heart is affected by the disease.
As with MS, corticosteroids are often prescribed, and may help maintain muscle strength. Once the disease progresses, wheelchairs and braces may be needed so that the patient can stay mobile. People with spinal problems related to MD may need surgery to make breathing easier. A pacemaker may also be required if the heart is affected by the disease.
Prognosis
Mild forms of multiple sclerosis do not necessarily affect life expectancy. Many people live 20 years or more with the disease, and those who develop it at a younger age often have the best outlook. People with more severe forms, with longer attacks and less time in remission, may not live as long. In rare cases, aggressive MS result in death in a very short amount of time.
People with more severe forms, with longer attacks and less time in remission, may not live as long. In rare cases, aggressive MS result in death in a very short amount of time.
The prognosis for someone with muscular dystrophy depends very much on which type the person has and how severe it is. Duchenne MD is typically fatal at a young age, and most people who have it do not live past their mid 20s. People with FSHD or myotonic MD, on the other hand, often live a normal life span.
Tricia has a Literature degree from Sonoma State University and has been a frequent contributor for many years. She is especially passionate about reading and writing, although her other interests include medicine, art, film, history, politics, ethics, and religion. Tricia lives in Northern California and is currently working on her first novel.
She is especially passionate about reading and writing, although her other interests include medicine, art, film, history, politics, ethics, and religion. Tricia lives in Northern California and is currently working on her first novel.
Tricia has a Literature degree from Sonoma State University and has been a frequent contributor for many years. She is especially passionate about reading and writing, although her other interests include medicine, art, film, history, politics, ethics, and religion. Tricia lives in Northern California and is currently working on her first novel.
You might also Like
Recommended
AS FEATURED ON:
Ocrelizumab significantly reduces the need for assistance with walking in relapsing multiple sclerosis and the risk of disability in primary progressive multiple sclerosis
Home > Industry news > Ocrelizumab Significantly Reduces Need for Assisted Walking in Relapsed MS and Risk of Disability in Primary Progressive MS - Evidence from an 8-Year Study
multiple sclerosis - data from an 8-year study
Roche presented data from long-term studies confirming the benefits of early initiation and long-term treatment with Ocrevus® (INN: ocrelizumab) in reducing the risk of disability in relapsing (RRMS) and primary progressive multiple sclerosis (PPMS), and safety outcomes for shortened two-hour infusions of ocrelizumab in minorities. Data from all clinical trials demonstrate a favorable benefit/risk profile of ocrelizumab therapy over eight years. Roche and study partners also released four latest abstracts to share the latest data on COVID-19and vaccine response in patients treated with ocrelizumab. The company presented these data at the 37th Congress of the European Committee for the Treatment and Research of Multiple Sclerosis. "Many neurologists have had direct experience with ocrelizumab for eight years in clinical trials and have observed consistently favorable results for its efficacy and safety in RRMS and PPMS, especially in reducing the risk of disability when given early in the disease," said Levi Garraway, Principal medical director and head of global product development at Roche. - New safety data from the shorter two-hour infusion is also encouraging, especially for populations that are often underrepresented in clinical trials. We remain committed to diversity and health equity in participation in clinical trials and access to treatment. The study also showed an association of switching to ocrelizumab early in the course of the disease with a rapid and sustained reduction in relapse rate per year (ARR) that was sustained through 5.5 years of open-label study. The ARR was 0.2 before switching, 0.1 after 1 year of ocrelizumab treatment, and 0.03 after 5.5 years of treatment in an open study. Subsequently, patients treated with ocrelizumab maintained a low ARR of 0.03 after 7.5 years of drug treatment. nine0003 Results of phase III open study ORATORIO : sustained reduction in overall and upper limb disability progression in primary progressive multiple sclerosis. Eight years of ocrelizumab results suggest a beneficial effect in slowing the progression of disability in patients with PPMS. The use of the drug at an early stage of the disease led to a decrease in the progression of disability by 29% at 48 weeks in patients with PPMS compared with patients who switched to ocrelizumab at 120 weeks (95% CI: 0.71 [0.57–0.87]; p = 0.001). The drug reduced the risk of recurrence of 48-week progressive disability by 24% in patients who continuously received ocrelizumab compared with those who switched from placebo (95% CI: 0.76 [0.62-0.92]; p = 0.005). Many people with PPMS end up using a wheelchair, so it is important for these patients to maintain the ability to use their hands. Upper limb disability progression as measured by the nine-hole test (9-HPT) also decreased in patients who continuously received ocrelizumab compared with those who switched from placebo (95% CI: 0.66 [0.50–0.86], respectively; p = 0.002).
Long-term safety data for ocrelizumab over 8 years New safety data as of November 2020 represent 5,688 patients with RMS and 21,675 patient-years of exposure to ocrelizumab across all clinical trials.
Faster Dosing Studies: Subgroup Analysis in Minority Populations The incidence and severity of drug-induced adverse events in Black, African American, Hispanic, and Hispanic populations treated with intravenous ocrelizumab for 2 hours were comparable to those events reported in the general patient population in subgroup analyzes of three studies (SaROD, CHORDS and ENSEMBLE PLUS). These patient populations may be susceptible to more severe disease and faster disease progression, despite being underreported in most clinical trials. Reducing the time of drug administration may ease the burden on these patient populations and allow them to access treatment. nine0003
Additional data: Impact of COVID -19 on patients treated with ocrelizumab Patient safety is Roche's top priority and we are closely monitoring the evolution of the COVID-19 pandemic. Four final annotations on the impact of the COVID-19 virus on patients treated with ocrelizumab, including response to vaccination, will be presented by Roche with research partners. At Roche, we are constantly striving to find new solutions for MS patients, and a faster two-hour twice-yearly (six-monthly) administration of ocrelizumab is now approved for patients with RMS or PPMS in the US and countries of the European Union (EU). nine0003 Ocrelizumab is approved in 97 countries in North America, South America, the Middle East, Eastern Europe, as well as Australia, Switzerland, the UK and the EU.
About multiple sclerosis Multiple sclerosis (MS) is a chronic disease that affects more than 2.8 million people worldwide. People with all types of MS are susceptible to disease progression—irreversible loss of nerve cells in the central nervous system and gradual worsening of disability—at the onset of the disease, even if clinical symptoms are not apparent or worsen. Delays in diagnosis and treatment can have negative consequences for MS patients in terms of their physical and mental health and financial situation. An important goal of MS treatment is to slow the progression of disability as early as possible. Relapsing-remitting multiple sclerosis (RRMS) is the most common form of the disease. It is characterized by exacerbations of neurological symptoms followed by periods of remission. Approximately 85% of people with MS are initially diagnosed with RRMS. Most patients diagnosed with RRMS develop secondary progressive MS (SPMS) over time, in which they continually experience worsening disability over time. Relapsing forms of MS (RRMS) include RMS and SPMS in which patients continue to experience exacerbations, or relapses. Primary progressive multiple sclerosis (PPMS) is a severe form of multiple sclerosis characterized by a constant increase in symptoms, usually without obvious exacerbations or periods of remission. This form accounts for approximately 15% of all cases of the disease. Prior to the approval of ocrelizumab, there were no FDA-approved treatments for PPMS. nine0003
About ocrelizumab
Roche in Neurology Neurology is a core area of research and development at Roche. Our goal is to advance the basic science to develop new drugs that will help improve the lives of patients with chronic and potentially fatal diseases. Roche develops more than a dozen drugs for the treatment of neurological diseases, including multiple sclerosis, Alzheimer's disease, Huntington's disease, Parkinson's disease, Duchenne muscular dystrophy and autism spectrum disorder. Together with our partners, we strive to push the boundaries of scientific understanding to solve some of the most challenging problems in the field of neuroscience. nine0003
About Roche Roche (Basel, Switzerland) is a global pharmaceutical and diagnostics innovation company that uses cutting-edge science to improve people's lives. Roche is one of the world's largest developers and manufacturers of biotech medicines for the treatment of cancer, autoimmune, infectious and neurological diseases. Roche is also one of the world leaders in the field of in vitro diagnostics, histological diagnostics of oncological diseases and a leading manufacturer of products for self-management of diabetes. nine0003 The synergy between pharmaceutical and diagnostics makes Roche a leading company in personalized medicine. In 2020, the company's investment in research and development amounted to 12.2 billion Swiss francs, with revenues of 58.3 billion Swiss francs. Roche owns Genentech, USA and a majority stake in Chugai Pharmaceutical, Japan. For more information visit www.roche.com. nine0003 Roche-Moscow JSC represents the pharmaceutical division of the company in Russia. Working with all stakeholders, we strive to improve the access of Russian patients to innovative technologies in the treatment of diseases. As of 2021, 30 of the company's drugs are included in the list of Vital and Essential Drugs. Roche makes a long-term contribution to the development of medicine, science, public health and the pharmaceutical industry in Russia. More details at www.roche.ru.
All trademarks mentioned above are protected by law. |
| Duchenne Muscular Dystrophy | Diagnosis and conservative treatment. Clinic prices, rating, reviews |
 5 years of use, according to the results of the opening phase III ( Ole )
5 years of use, according to the results of the opening phase III ( Ole ) 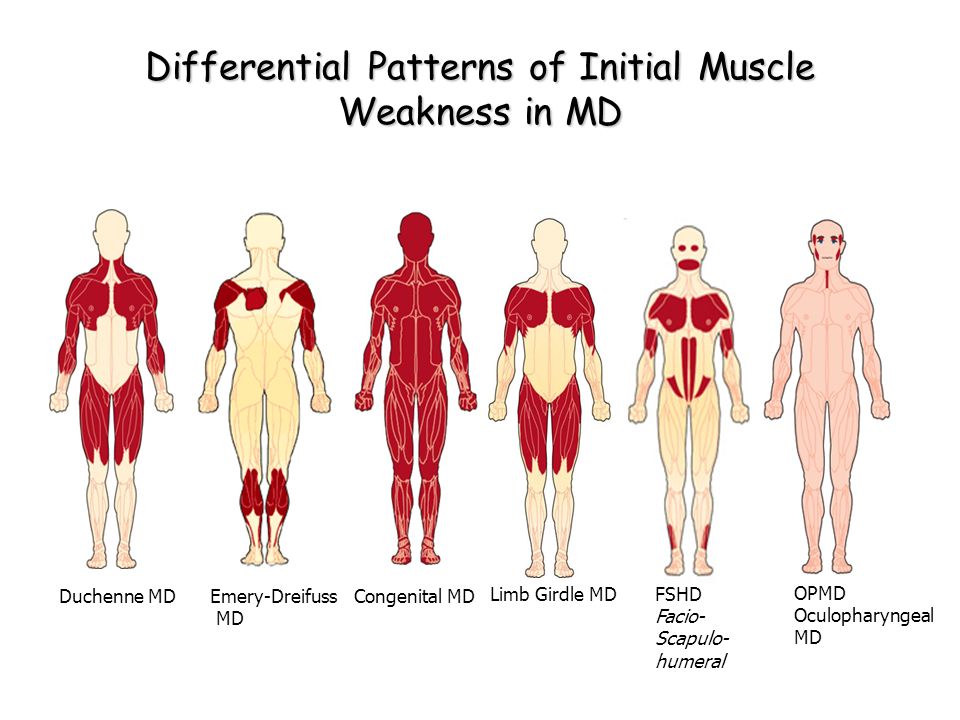
 ” nine6-week participation in a double-blind study (5.2% vs. 7.0%, respectively; 95% CI: 0.65 [0.44–0.97]; p = 0.034). Risk is measured by the time period during which a person achieved a score of 6 or more on the disability status scale, which persisted for at least 48 weeks in the post-hoc analysis.
” nine6-week participation in a double-blind study (5.2% vs. 7.0%, respectively; 95% CI: 0.65 [0.44–0.97]; p = 0.034). Risk is measured by the time period during which a person achieved a score of 6 or more on the disability status scale, which persisted for at least 48 weeks in the post-hoc analysis. 
 These results further demonstrate a consistently favorable benefit/risk ratio of the drug over eight years. nine0003
These results further demonstrate a consistently favorable benefit/risk ratio of the drug over eight years. nine0003 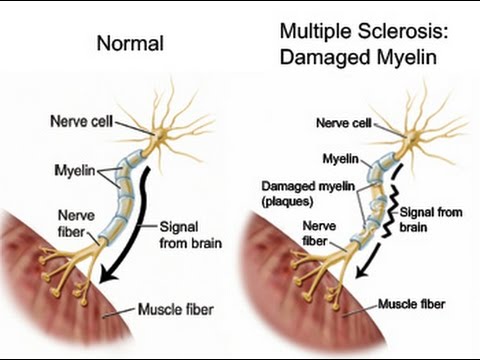 We are committed to working closely with the community to better understand the impact of the COVID-19 virus on people treated with ocrelizumab and will continue to share new data with MS patients as it becomes available. nine0003
We are committed to working closely with the community to better understand the impact of the COVID-19 virus on people treated with ocrelizumab and will continue to share new data with MS patients as it becomes available. nine0003 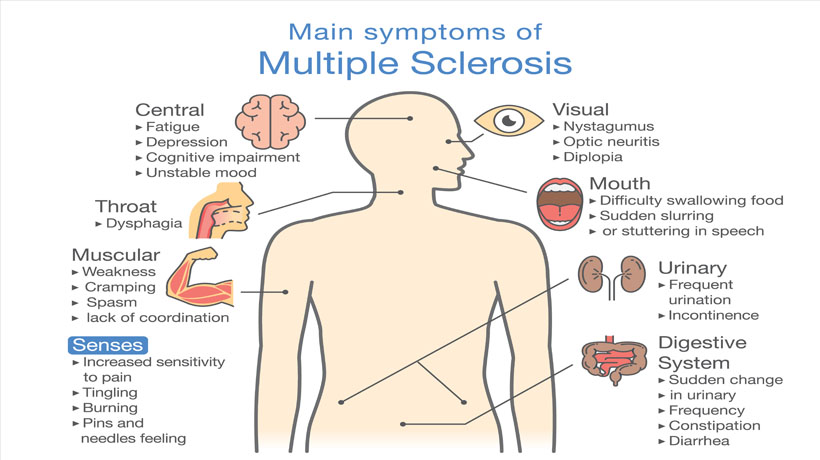 MS occurs when the immune system attacks the myelin sheath of nerve fibers in the central nervous system (brain, spinal cord, and optic nerves), causing inflammation and subsequent damage. This damage can cause a wide range of symptoms, including muscle weakness, fatigue, and vision problems, and can eventually lead to disability. Most people with MS experience their first symptom between the ages of 20 and 40, making the disease the leading cause of non-traumatic disability in young adults. nine0003
MS occurs when the immune system attacks the myelin sheath of nerve fibers in the central nervous system (brain, spinal cord, and optic nerves), causing inflammation and subsequent damage. This damage can cause a wide range of symptoms, including muscle weakness, fatigue, and vision problems, and can eventually lead to disability. Most people with MS experience their first symptom between the ages of 20 and 40, making the disease the leading cause of non-traumatic disability in young adults. nine0003  nine0003
nine0003  Ocrelizumab is a humanized monoclonal antibody designed to target CD20-positive B cells, a special type of immune cell thought to be the main cause of damage to myelin (the insulating and supporting substance of nerve cells) and axons (nerve cells). Damage to nerve cells can lead to disability in people with multiple sclerosis. Based on preclinical studies, ocrelizumab binds to CD20 cell surface proteins expressed on certain B cells, but not on stem or plasma cells, suggesting that important immune system functions may be preserved. Ocrelizumab is given intravenously every six months. The initial dose is two infusions of 300 mg with an interval of two weeks. Subsequent doses are administered as single infusions of 600 mg. nine0003
Ocrelizumab is a humanized monoclonal antibody designed to target CD20-positive B cells, a special type of immune cell thought to be the main cause of damage to myelin (the insulating and supporting substance of nerve cells) and axons (nerve cells). Damage to nerve cells can lead to disability in people with multiple sclerosis. Based on preclinical studies, ocrelizumab binds to CD20 cell surface proteins expressed on certain B cells, but not on stem or plasma cells, suggesting that important immune system functions may be preserved. Ocrelizumab is given intravenously every six months. The initial dose is two infusions of 300 mg with an interval of two weeks. Subsequent doses are administered as single infusions of 600 mg. nine0003 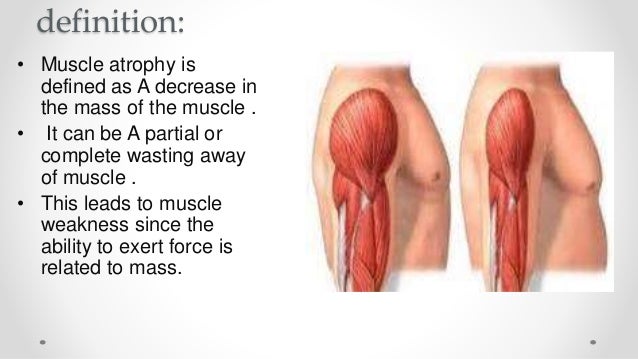
 For 11 consecutive years, Roche has been recognized as a leader in the pharmaceutical industry for sustainable development, according to the Dow Jones index.
For 11 consecutive years, Roche has been recognized as a leader in the pharmaceutical industry for sustainable development, according to the Dow Jones index.  nine0003
nine0003 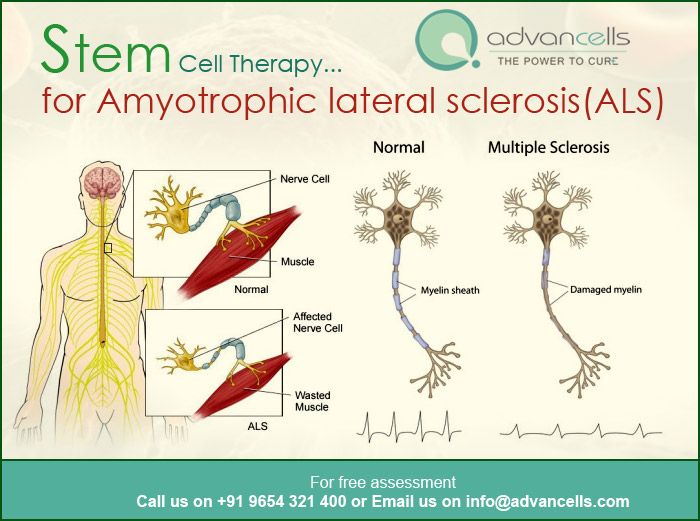 The department specializes in the treatment of epilepsy, headaches, migraine and other neurological disorders. For diagnostics, there is the latest equipment, for example, the 3T MRI machine, which makes it possible to detect changes in the cerebral cortex, which are a possible cause of epilepsy. nine0003
The department specializes in the treatment of epilepsy, headaches, migraine and other neurological disorders. For diagnostics, there is the latest equipment, for example, the 3T MRI machine, which makes it possible to detect changes in the cerebral cortex, which are a possible cause of epilepsy. nine0003 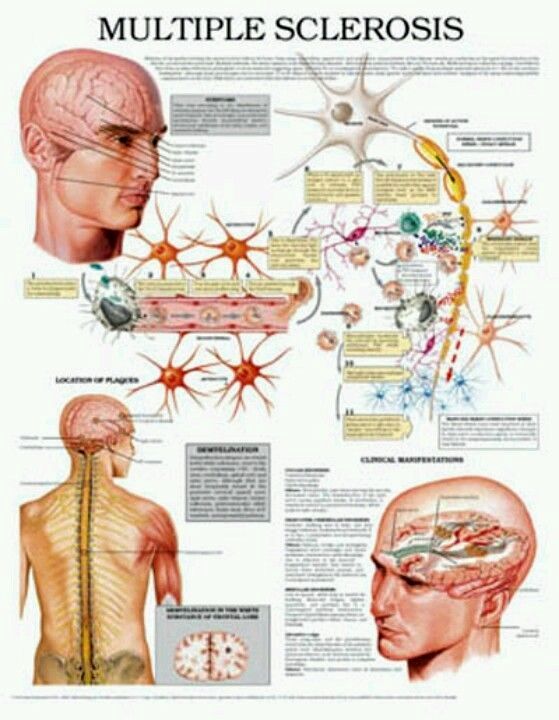 nine0003
nine0003 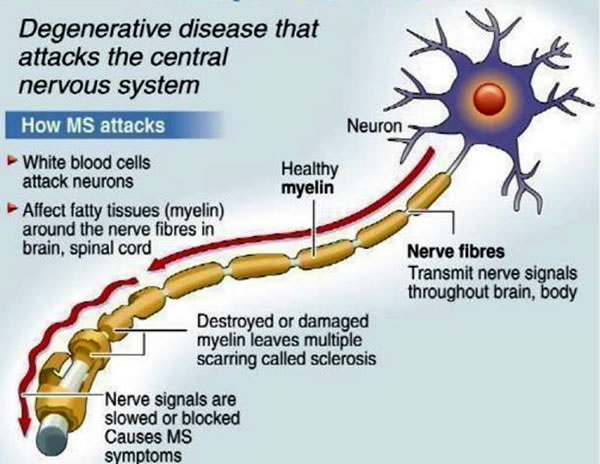 The department has a friendly atmosphere, and all conditions are created for the provision of high-quality medical care. According to the authoritative American news publication Newsweek, the department was included in the ranking of the best neurological centers in the world. nine0003
The department has a friendly atmosphere, and all conditions are created for the provision of high-quality medical care. According to the authoritative American news publication Newsweek, the department was included in the ranking of the best neurological centers in the world. nine0003 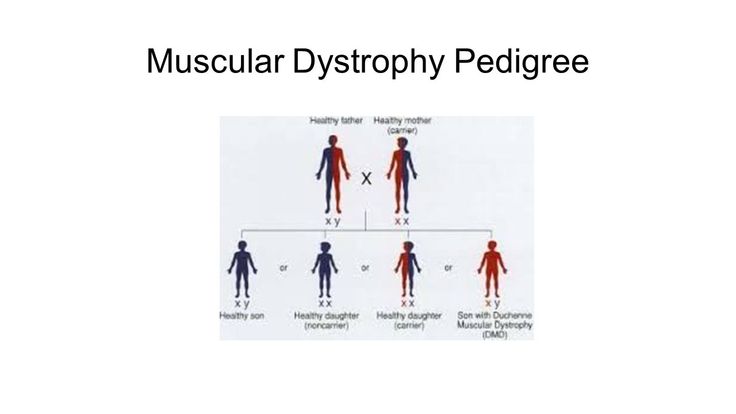 In addition, the department includes a certified stroke unit. A total of 71 beds are available at the facility to accommodate inpatients. The goal of all specialists of the department is to provide optimal therapy for a sustainable improvement in the quality of life and health of patients with neurological diseases. nine0003
In addition, the department includes a certified stroke unit. A total of 71 beds are available at the facility to accommodate inpatients. The goal of all specialists of the department is to provide optimal therapy for a sustainable improvement in the quality of life and health of patients with neurological diseases. nine0003 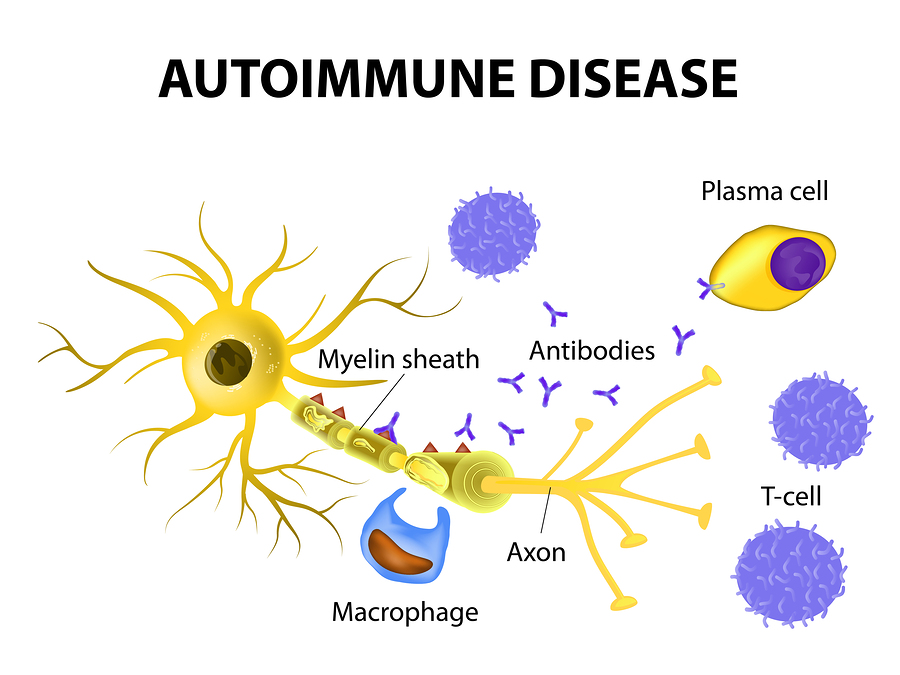 This is of particular importance in the treatment of complex and rare forms of neurological diseases. nine0003
This is of particular importance in the treatment of complex and rare forms of neurological diseases. nine0003 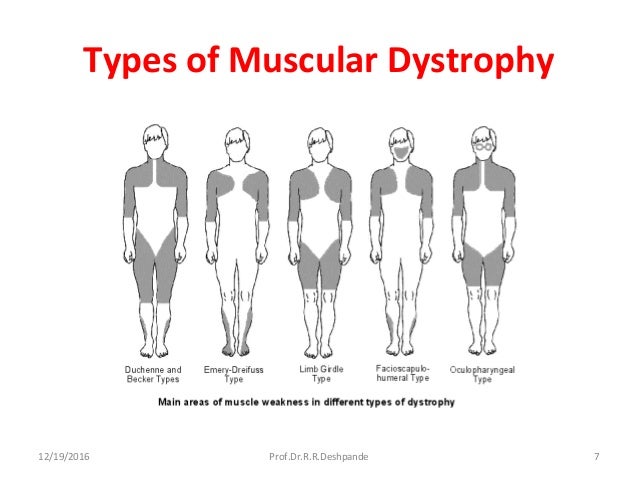 8/10of 57 reviews
8/10of 57 reviews  Patient care is provided both on an outpatient and inpatient basis. Of particular interest is the treatment of movement disorders, epilepsy, neuro-oncological pathologies, stroke and other ailments. The department employs highly qualified doctors who not only guarantee the most effective treatment, but also surround the patient with care, demonstrate a respectful attitude and understanding of the needs of each patient. nine0003
Patient care is provided both on an outpatient and inpatient basis. Of particular interest is the treatment of movement disorders, epilepsy, neuro-oncological pathologies, stroke and other ailments. The department employs highly qualified doctors who not only guarantee the most effective treatment, but also surround the patient with care, demonstrate a respectful attitude and understanding of the needs of each patient. nine0003 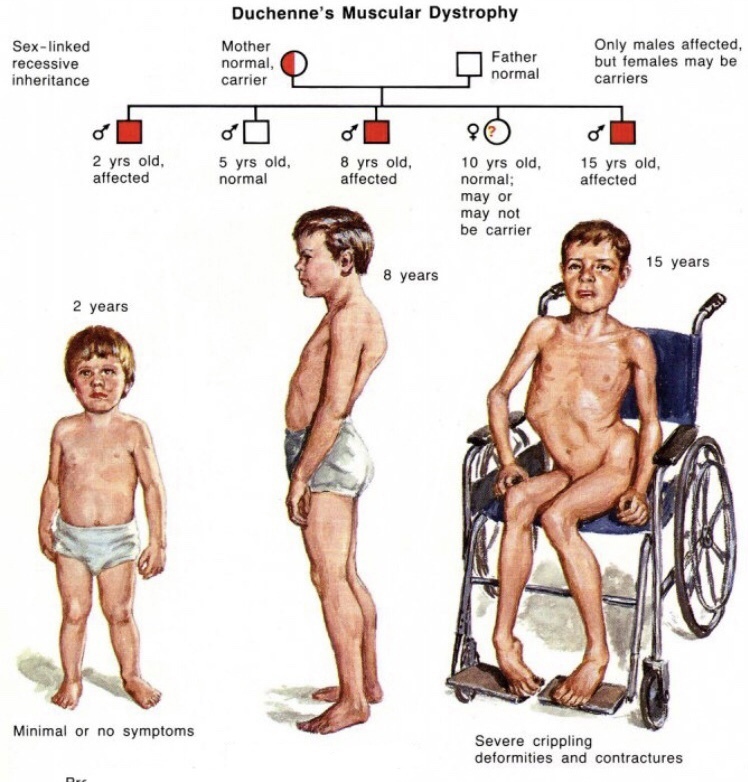 A specialized stroke unit operates here to provide emergency care to patients with stroke. Epilepsy treatment is carried out in a specialized center - one of the best in all of Germany. The success of treatment is largely determined by the quality of the preliminary examination, therefore, the department has an advanced technical base that allows for a comprehensive examination of the nervous system and reveals the slightest functional and structural disorders. Specialists in the field of neurosurgery and neuroradiology are actively involved in the therapeutic process, who regularly meet at consultations to consider complex clinical cases. With more than 3,500 inpatients and about 7,000 outpatients annually, the department is one of the largest and most successful in its field of specialization in the country. nine0003
A specialized stroke unit operates here to provide emergency care to patients with stroke. Epilepsy treatment is carried out in a specialized center - one of the best in all of Germany. The success of treatment is largely determined by the quality of the preliminary examination, therefore, the department has an advanced technical base that allows for a comprehensive examination of the nervous system and reveals the slightest functional and structural disorders. Specialists in the field of neurosurgery and neuroradiology are actively involved in the therapeutic process, who regularly meet at consultations to consider complex clinical cases. With more than 3,500 inpatients and about 7,000 outpatients annually, the department is one of the largest and most successful in its field of specialization in the country. nine0003  The department specializes in the treatment of Parkinson's disease and other movement disorders. The department also offers the latest methods for the diagnosis and treatment of multiple sclerosis, vascular diseases of the central nervous system, neuromuscular diseases, various forms of epilepsy, etc. In addition, the department provides emergency care to stroke patients in a specialized stroke unit. nine0003
The department specializes in the treatment of Parkinson's disease and other movement disorders. The department also offers the latest methods for the diagnosis and treatment of multiple sclerosis, vascular diseases of the central nervous system, neuromuscular diseases, various forms of epilepsy, etc. In addition, the department provides emergency care to stroke patients in a specialized stroke unit. nine0003 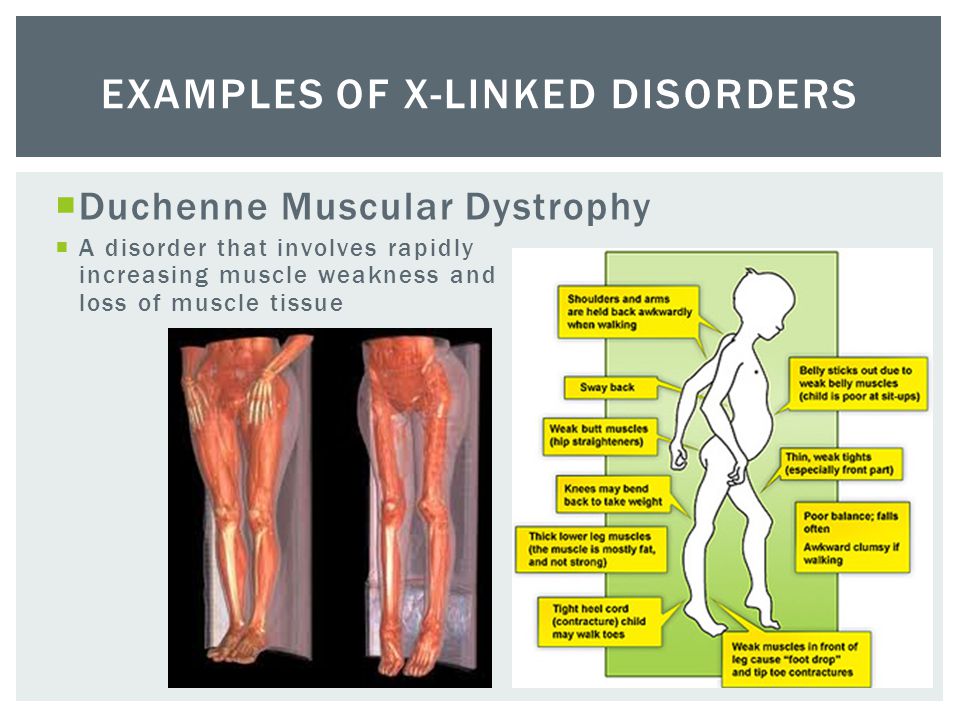 Thus, the department has created all the conditions for providing first-class neurological care. nine0003
Thus, the department has created all the conditions for providing first-class neurological care. nine0003  Particular attention is paid to the treatment of epilepsy, neurodegenerative diseases, neurovascular pathologies, oncoprocesses of the nervous system and cognitive disorders. To make an accurate diagnosis, the department has access to all modern diagnostic capabilities, such as Doppler scanning, electroencephalography, electromyography, electronystagmography and other highly specialized studies. nine0003
Particular attention is paid to the treatment of epilepsy, neurodegenerative diseases, neurovascular pathologies, oncoprocesses of the nervous system and cognitive disorders. To make an accurate diagnosis, the department has access to all modern diagnostic capabilities, such as Doppler scanning, electroencephalography, electromyography, electronystagmography and other highly specialized studies. nine0003  On the basis of the department, an ultramodern stroke unit operates, whose specialists can quickly and efficiently diagnose stroke in the acute phase and take appropriate therapeutic measures. The stroke unit operates around the clock. nine0003
On the basis of the department, an ultramodern stroke unit operates, whose specialists can quickly and efficiently diagnose stroke in the acute phase and take appropriate therapeutic measures. The stroke unit operates around the clock. nine0003