Can gabapentin be used for anxiety
Treatment of Generalized Anxiety Disorder with Gabapentin
- Journal List
- Case Rep Psychiatry
- v.2017; 2017
- PMC5745655
Case Rep Psychiatry. 2017; 2017: 6045017.
Published online 2017 Dec 14. doi: 10.1155/2017/6045017
and
Author information Article notes Copyright and License information Disclaimer
Gabapentin is frequently used in the treatment of anxiety disorders. However, there are no randomized controlled trials on the effectiveness of this medication in generalized anxiety disorder (GAD), and there are only a few case reports. We present a case of a 59-year-old female with a psychiatric history of GAD.
The patient discontinued benzodiazepines after more than 7 years of daily treatment which led to rebound anxiety, benzodiazepine withdrawal symptoms, and suicidal ideation. She was psychiatrically hospitalized and started on gabapentin. Over the next 10 months of outpatient follow-up, she attempted to taper off gabapentin due to personal preference to limit medications. During this time, we observed a clear dose-response pattern of gabapentin on GAD symptoms. In the absence of controlled studies, these findings may offer important information about the effectiveness of gabapentin in GAD.
While gabapentin is increasingly being used to treat generalized anxiety disorder (GAD), little is known about its effectiveness on GAD symptoms. The patient presented here has a relatively straightforward psychiatric history, with GAD playing a prominent role. Her repeated attempts to discontinue gabapentin offer a rare opportunity to observe its effect on her symptoms at different doses. A clear pattern of remission or mild anxiety on total daily doses of gabapentin ≥ 900 mg/day and severe anxiety at doses < 600 mg/day was observed. In the absence of randomized controlled trials, these findings may offer clinically important clues about dosing and effectiveness of gabapentin in GAD.
In the absence of randomized controlled trials, these findings may offer clinically important clues about dosing and effectiveness of gabapentin in GAD.
2.1. Presenting Features
The patient is a 59-year-old Caucasian female. Her psychiatric history is significant for major depressive disorder (MDD) and GAD. She has had four prior inpatient psychiatric hospitalizations for depression and anxiety with suicidality.
Her medical history is pertinent for Hashimoto's thyroiditis with primary hypothyroidism on levothyroxine, primary Sjögren's syndrome (asymptomatic), and autoimmune diabetes with peripheral neuropathy. Social history is pertinent for physical and emotional abuse in childhood. She does not use alcohol, tobacco, or illicit substances. She drinks up to two cups of caffeinated coffee in the morning. Her family history is positive for MDD, GAD, and bipolar disorder.
In the past, the patient failed or did not tolerate treatment with multiple selective serotonin reuptake inhibitors (SSRIs; citalopram up to 100 mg/day, paroxetine 30 mg/day), serotonin-norepinephrine reuptake inhibitors (SNRIs; duloxetine up to 90 mg/day), tricyclic antidepressants (amitriptyline at therapeutic levels and 10 mg/day nortriptyline), mirtazapine 30 mg/day, bupropion 150 mg/day, aripiprazole (discontinued due to concern for excessive gambling), and trazodone.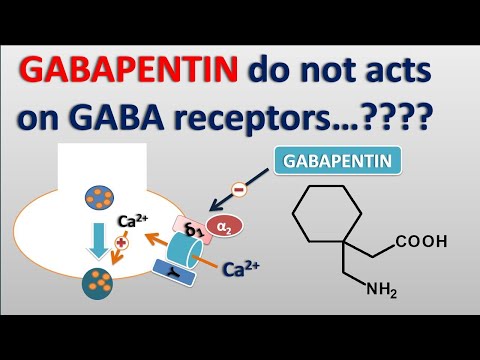 Pregabalin 150 mg/day was helpful for anxiety in the past. She had multiple unsuccessful cognitive behavioral therapy trials. At the age of 52, she was prescribed clonazepam on an “as needed basis” for mild GAD (started on 0.5 mg two times per day (BID) and gradually increased to 1 mg BID).
Pregabalin 150 mg/day was helpful for anxiety in the past. She had multiple unsuccessful cognitive behavioral therapy trials. At the age of 52, she was prescribed clonazepam on an “as needed basis” for mild GAD (started on 0.5 mg two times per day (BID) and gradually increased to 1 mg BID).
Three months prior to presentation, benzodiazepines were tapered and the patient's anxiety recurred. Her symptoms included persistent and excessive worry, restlessness, feeling on edge, fatigue, poor concentration, irritability, insomnia, and dysphoric mood, as well as diaphoresis, palpitations, tremulousness, and muscle tension. She was unable to complete activities of daily living. She developed suicidal ideation and required two psychiatric hospitalizations in a one-month span. Ultimately, benzodiazepines were successfully discontinued and she has remained off of these medications throughout 10 months of outpatient follow-up.
During her most recent hospitalization, gabapentin was initiated at a dose of 300 mg three times per day (TID) to manage benzodiazepine withdrawal symptoms. Although she reported some sedation with this dose, she felt “calmer” and her anxiety dissipated. Her dose was titrated to 600 mg TID, and she was discharged from the hospital.
Although she reported some sedation with this dose, she felt “calmer” and her anxiety dissipated. Her dose was titrated to 600 mg TID, and she was discharged from the hospital.
2.2. Differential Diagnoses
We considered generalized anxiety disorder, panic disorder, posttraumatic stress disorder, MDD, obsessive compulsive disorder, anxiety due to another medical condition, such as thyroid disease, medication-induced mood, and anxiety disorder from prolonged benzodiazepine withdrawal.
2.3. Progression, Treatment, Follow-Up, and Outcome
Apart from gabapentin 600 mg TID, the patient's other discharge medications included doxepin 10 mg at bedtime for sleep, hydroxyzine pamoate 25 mg BID as needed for anxiety, and sertraline 100 mg daily, all of which were discontinued due to side effects or ineffectiveness during subsequent outpatient follow-up. During her outpatient follow-up, anxiety was assessed using a combination of Likert-scale ratings and the generalized anxiety disorder 7-item (GAD-7) patient self-report questionnaire. Four days after discharge, sertraline was increased to 150 mg as per hospital recommendations. She did not tolerate hydroxyzine on as needed basis and stopped using this medication several days after leaving the hospital.
Four days after discharge, sertraline was increased to 150 mg as per hospital recommendations. She did not tolerate hydroxyzine on as needed basis and stopped using this medication several days after leaving the hospital.
2.3.1. First Gabapentin Discontinuation Attempt
On day 18 after discharge, she attempted a self-initiated gabapentin taper due to improved anxiety and perceived “drowsiness” (see for graphical depiction of anxiety-gabapentin dose relationship over time). She decreased the dose to 300 mg TID with no problematic change in her anxiety. On day 30, she requested to stop gabapentin as her anxiety was well controlled. Her outpatient psychiatrist recommended a slow taper, so gabapentin was decreased to 300 mg BID for 1 week. However, her anxiety rapidly returned. Nevertheless, she further decreased gabapentin to 300 mg at bedtime (QHS). At this point, her anxiety became debilitating. On day 48 following discharge, one month after her first self-initiated dose reduction, her gabapentin was again increased to 300 mg TID with immediate and pronounced decrease in anxiety ().
Open in a separate window
Relationship between gabapentin dose and anxiety. Anxiety was assessed on a 0–10 scale; GAD-7 scores are added where available. Day 0 is the day of discharge from the last hospitalization. Gabapentin is expressed in total daily doses. Measurements were not continuous, but for clarity lines connect the values obtained during in-office follow-up appointments or telephone conversations (a total of 27 measurements in 294 days).
2.3.2. Second Gabapentin Discontinuation Attempt
The patient remained on 300 mg TID for approximately one month; however, by day 75, she initiated a second gabapentin self-taper with a reduction to 300 mg BID because she believed that gabapentin was causing her somnolence. She further reduced her dose to 300 mg in the morning and 150 mg QHS on day 77. This change was again accompanied by increased anxiety within days, and 19 days after the last dose reduction, her gabapentin was returned to 300 mg BID with immediate improvement in anxiety ().
2.3.3. Third Gabapentin Discontinuation Attempt
On day 118, the patient attempted a third self-initiated gabapentin taper as she believed gabapentin was causing increased fatigue. She first reduced her dose to 300 mg for one week and then further to 150 mg daily for a period of 10 days. However, this was accompanied by another increase in anxiety, and her dose was increased to 300 mg daily. At the same time, sertraline was cross-tapered to amitriptyline to treat chronic migraine. Amitriptyline was titrated to a dose of 50 mg/day, where she achieved a total amitriptyline + nortriptyline concentration of 101 ng/mL [ref.: 80–200 ng/mL]. Doxepin 10 mg was discontinued. Her headache temporarily resolved, but anxiety continued to impact her functioning. During the 2 weeks, while the above-mentioned medication changes were taking place, her anxiety has been increasing but staying in the moderate range on gabapentin 300 mg. As per her request, her dose was increased to 300 mg BID on day 149, followed by a decrease in her anxious symptoms within 24 hours ().
2.3.4. Period of Stability
Gabapentin was subsequently continued at a dose of 300 mg BID for approximately 1 month with mild but manageable symptoms present. However, shortly thereafter, the patient had a number of psychosocial stressors that led to increased anxiety (GAD-7 score of 19). Gabapentin was increased to 600 mg BID, and after approximately one month amitriptyline was discontinued as the patient was not convinced of its benefits. Her anxiety decreased to 0/10 within 48 hours of increasing gabapentin, and she remained in remission on gabapentin monotherapy for the next 70 days despite ongoing psychosocial stressors. At the end of this period, she started to develop some depressive symptoms (Patient Health Questionnaire 9 score of 17), though her anxiety remained mild. Venlafaxine was therefore initiated. The patient remains on gabapentin and venlafaxine at the time of manuscript submission.
The potential anxiolytic effect of gabapentin was first observed in animal models [1]. Randomized controlled trials in patients with anxiety disorders found that gabapentin is effective in treating social phobia [2]. Gabapentin was generally not effective in treatment of panic and agoraphobia symptoms. However, a subgroup of more severely ill patients, particularly women, did show some improvement [3].
Randomized controlled trials in patients with anxiety disorders found that gabapentin is effective in treating social phobia [2]. Gabapentin was generally not effective in treatment of panic and agoraphobia symptoms. However, a subgroup of more severely ill patients, particularly women, did show some improvement [3].
To our knowledge, there are no controlled studies on gabapentin use in GAD [4]. A compiled study of 18 patients with various anxiety disorders, one of which had GAD, found beneficial effects on anxiety symptoms [5]. Pollack and colleagues published a case report of a patient with GAD who improved on gabapentin 100 mg TID at 3-month follow-up [6]. However, the patient was also receiving 20 mg total daily dose of diazepam [6]. The same author also reported improved anxiety in a patient receiving gabapentin 100 mg BID at 3-month follow-up [6]. However, that patient had alcohol use disorder and had decreased drinking during this period [6]. Despite this lack of efficacy data, our clinical experience indicates that gabapentin is frequently used to treat patients with GAD.
The patient presented here has the most detailed description of gabapentin dose-response on GAD symptoms available in the literature thus far. On average, we contacted the patient every 11 days, for a total of 27 measurements in 294 days of outpatient follow-up. This allows us to determine a detailed dose-efficacy response for this patient. As shown in , there was a clear inverse relationship between gabapentin dose and anxiety. Anxiety was rated as low or absent at total daily doses ≥ 900 mg per day. This is particularly encouraging as the patient had multiple failed therapeutic trials of SSRIs, SNRIs, tricyclic antidepressants, bupropion, mirtazapine, aripiprazole, and trazodone targeting anxiety and mood. Compared to these medications, gabapentin has a favorable side effect profile and generally represents lower risk in overdose [7].
The patient was discharged from her last hospitalization on several psychotropic medications including gabapentin, sertraline, doxepin 10 mg QHS, and hydroxyzine 25 mg BID. She quickly discontinued hydroxyzine but continued the other three medications until day 124. During this time, gabapentin was the only medication with repeated dose adjustments, and no other medication changes were temporally correlated with the fluctuating levels of anxiety in this patient. Despite some degree of polypharmacy, the patient's fluctuating anxiety levels were therefore most clearly associated with gabapentin dose changes. Furthermore, starting on day 196, the patient's psychiatric condition was treated with gabapentin 600 mg BID monotherapy for approximately 70 days. She remained in complete remission from anxiety during this period, further supporting the hypothesis that gabapentin at total daily doses ≥ 900 mg/day was effective in treating her GAD symptoms. At the end of this period, the patient developed symptoms consistent with MDD, warranting initiation of venlafaxine; however, she showed no signs of anxious distress, again consistent with gabapentin's efficacy in managing her anxiety symptoms.
She quickly discontinued hydroxyzine but continued the other three medications until day 124. During this time, gabapentin was the only medication with repeated dose adjustments, and no other medication changes were temporally correlated with the fluctuating levels of anxiety in this patient. Despite some degree of polypharmacy, the patient's fluctuating anxiety levels were therefore most clearly associated with gabapentin dose changes. Furthermore, starting on day 196, the patient's psychiatric condition was treated with gabapentin 600 mg BID monotherapy for approximately 70 days. She remained in complete remission from anxiety during this period, further supporting the hypothesis that gabapentin at total daily doses ≥ 900 mg/day was effective in treating her GAD symptoms. At the end of this period, the patient developed symptoms consistent with MDD, warranting initiation of venlafaxine; however, she showed no signs of anxious distress, again consistent with gabapentin's efficacy in managing her anxiety symptoms.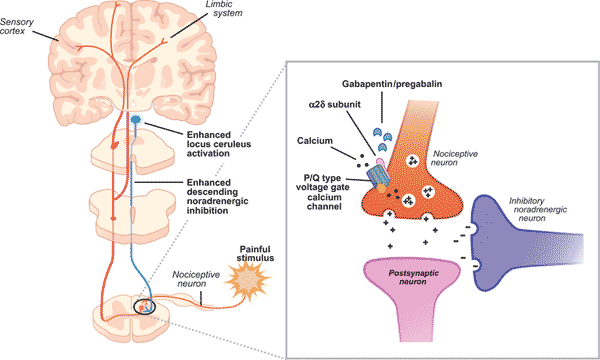
In addition to medication concerns, this patient had a particularly high autoimmune disease burden including Hashimoto's thyroiditis, primary Sjögren's syndrome, and autoimmune diabetes. Little is known about the interaction between gabapentin and autoimmune mechanisms. Worsening of myasthenia gravis with gabapentin has been reported, and one case of gabapentin-induced bullous pemphigoid is currently available in the literature [8, 9]. To our knowledge, there have been no controlled studies or case reports indicating that patients with a high autoimmune disease burden should have an altered mood or anxiety response to gabapentin. However, this is a potential consideration that deserves further research. Overall, we believe that no convincing evidence exists at this time to suggest that this patient's favorable response to gabapentin is linked to her autoimmune predisposition or that using gabapentin in such a patient carries an increased risk of adverse events.
We did not measure gabapentin's serum levels in this patient. Firstly, the target serum concentration for treating anxiety with gabapentin is not known and therefore is not clinically useful at this point. Secondly, there was no evidence that the patient falsely reported her gabapentin use, as she was forthcoming with her psychiatrist about her repeated episodes of self-initiated dose reductions and periods of taking the medication as prescribed. Additionally, medication refills were requested and filled at the expected times for the doses that she was taking.
Firstly, the target serum concentration for treating anxiety with gabapentin is not known and therefore is not clinically useful at this point. Secondly, there was no evidence that the patient falsely reported her gabapentin use, as she was forthcoming with her psychiatrist about her repeated episodes of self-initiated dose reductions and periods of taking the medication as prescribed. Additionally, medication refills were requested and filled at the expected times for the doses that she was taking.
Gabapentin has a complex mechanism of action that only modestly overlaps with other antiepileptics. Its effect is likely mediated partially through inhibition of voltage-gated calcium channels via binding to α2δ-1 subunits [10]. This affects cellular trafficking of voltage-dependent calcium channels likely causing an overall reduction in calcium currents and potentially impacting neurotransmitter release in currently unknown ways [10]. Binding to this receptor complex has also been postulated to decrease the formation of excitatory neuronal synapses, thus potentially decreasing overall excitatory tone and modulating anxiety [11]. Other potential mechanisms of action that may target anxiety symptoms include modulation of GABA biosynthesis and nonsynaptic GABA neurotransmission [10, 12]. It is notable that although gabapentin does not directly bind to or modulate GABA-A receptors like benzodiazepines, the above mechanisms may indirectly impact GABAergic tone and provide a treatment option not only for anxiety but also for components of both benzodiazepine and alcohol withdrawal [10, 13, 14]. While this patient indeed found gabapentin useful for her benzodiazepine discontinuation, a larger study found that gabapentin was not associated with decreased benzodiazepine use in psychiatric patients [15]. We hypothesize that it was gabapentin's efficacy in treating GAD symptoms that allowed for ongoing benzodiazepine abstinence. Future research is necessary to determine whether patients with GAD who respond positively to gabapentin also have decreased benzodiazepine utilization. This is particularly important given the risks of benzodiazepines in older adults [16].
Other potential mechanisms of action that may target anxiety symptoms include modulation of GABA biosynthesis and nonsynaptic GABA neurotransmission [10, 12]. It is notable that although gabapentin does not directly bind to or modulate GABA-A receptors like benzodiazepines, the above mechanisms may indirectly impact GABAergic tone and provide a treatment option not only for anxiety but also for components of both benzodiazepine and alcohol withdrawal [10, 13, 14]. While this patient indeed found gabapentin useful for her benzodiazepine discontinuation, a larger study found that gabapentin was not associated with decreased benzodiazepine use in psychiatric patients [15]. We hypothesize that it was gabapentin's efficacy in treating GAD symptoms that allowed for ongoing benzodiazepine abstinence. Future research is necessary to determine whether patients with GAD who respond positively to gabapentin also have decreased benzodiazepine utilization. This is particularly important given the risks of benzodiazepines in older adults [16].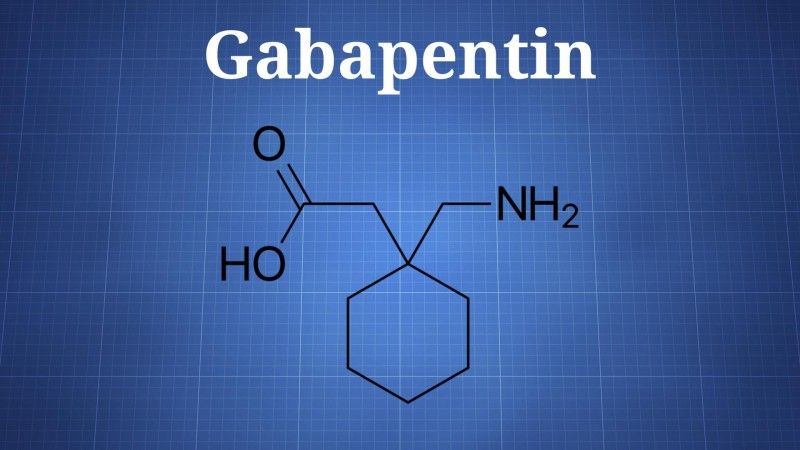
There has been growing concern about the potential for gabapentin abuse. However, the literature is limited and suggests that gabapentin is predominantly abused by patients with other substance use disorders, notably opioid use disorder [17–21]. The doses used when gabapentin is abused tend to be higher than 3000 mg/day [17]. Our patient showed no signs of abuse or increasing demand for gabapentin and seems to be low risk based on available literature. Nevertheless, it is important for physicians to be aware of the potential for gabapentin abuse when considering prescribing it, particularly for patients with substance use disorders.
Informed consent for the publication of this manuscript has been obtained from the patient.
The authors declare that there are no conflicts of interest regarding the publication of this paper.
1. Singh L., Field M. J., Ferris P., et al. The antiepileptic agent gabapentin (Neurontin) possesses anxiolytic-like and antinociceptive actions that are reversed by D-serine. Psychopharmacology. 1996;127(1):1–9. doi: 10.1007/BF02805968. [PubMed] [CrossRef] [Google Scholar]
Psychopharmacology. 1996;127(1):1–9. doi: 10.1007/BF02805968. [PubMed] [CrossRef] [Google Scholar]
2. Pande A. C., Davidson J. R. T., Jefferson J. W., et al. Treatment of social phobia with gabapentin: A placebo-controlled study. Journal of Clinical Psychopharmacology. 1999;19(4):341–348. doi: 10.1097/00004714-199908000-00010. [PubMed] [CrossRef] [Google Scholar]
3. Pande A. C., Pollack M. H., Crockatt J., et al. Placebo-controlled study of gabapentin treatment of panic disorder. Journal of Clinical Psychopharmacology. 2000;20(4):467–471. doi: 10.1097/00004714-200008000-00011. [PubMed] [CrossRef] [Google Scholar]
4. Berlin R. K., Butler P. M., Perloff M. D. Gabapentin therapy in psychiatric disorders: A systematic review. Primary Care Companion to the Journal of Clinical Psychiatry. 2015;17(5) doi: 10.4088/PCC.15r01821. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
5. Chouinard G., Beauclair L., Belanger M.-C. Gabapentin: Long-term antianxiety and hypnotic effects in psychiatric patients with comorbid anxiety-related disorders. The Canadian Journal of Psychiatry. 1998;43(3, article 305) doi: 10.1177/070674379804300315. [PubMed] [CrossRef] [Google Scholar]
The Canadian Journal of Psychiatry. 1998;43(3, article 305) doi: 10.1177/070674379804300315. [PubMed] [CrossRef] [Google Scholar]
6. Pollack M. H., Matthews J., Scott E. L. Gabapentin as a potential treatment for anxiety disorders. The American Journal of Psychiatry. 1998;155(7):992–993. doi: 10.1176/ajp.155.7.992. [PubMed] [CrossRef] [Google Scholar]
7. Wills B., Reynolds P., Chu E., et al. Clinical outcomes in newer anticonvulsant overdose: a poison center observational study. Journal of Medical Toxicology. 2014;10(3):254–260. doi: 10.1007/s13181-014-0384-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
8. Boneva N., Brenner T., Argov Z. Gabapentin may be hazardous in myasthenia gravis. Muscle & Nerve. 2000;23(8):1204–1208. doi: 10.1002/1097-4598(200008)23:8<1204::AID-MUS7>3.0.CO;2-H. [PubMed] [CrossRef] [Google Scholar]
9. Flamm A., Sachdev S., Dufresne F. Gabapentin-induced bullous pemphigoid. The Journal of the American Osteopathic Association. 2017;117(3):191–193. doi: 10.7556/jaoa.2017.034. [PubMed] [CrossRef] [Google Scholar]
2017;117(3):191–193. doi: 10.7556/jaoa.2017.034. [PubMed] [CrossRef] [Google Scholar]
10. Sills G. The mechanisms of action of gabapentin and pregabalin. Current Opinion in Pharmacology. 2006;6(1):108–113. doi: 10.1016/j.coph.2005.11.003. [PubMed] [CrossRef] [Google Scholar]
11. Eroglu Ç., Allen N. J., Susman M. W., et al. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380–392. doi: 10.1016/j.cell.2009.09.025. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
12. Taylor C. P. Mechanisms of action of gabapentin. Revue Neurologique. 1997;(suppl. 1):S39–S45. [PubMed] [Google Scholar]
13. Jenner P., Pratt J. A., Marsden C. D. Mechanism of action of clonazepam in myoclonus in relation to effects on GABA and 5-HT. Advances in Neurology. 1986;43:629–643. [PubMed] [Google Scholar]
14. Leung J. G., Hall-Flavin D., Nelson S., Schmidt K. A., Schak K. M. Role of gabapentin in the management of alcohol withdrawal and dependence. Annals of Pharmacotherapy. 2015;49(8):897–906. doi: 10.1177/1060028015585849. [PubMed] [CrossRef] [Google Scholar]
A., Schak K. M. Role of gabapentin in the management of alcohol withdrawal and dependence. Annals of Pharmacotherapy. 2015;49(8):897–906. doi: 10.1177/1060028015585849. [PubMed] [CrossRef] [Google Scholar]
15. Bramness J. G., Sandvik P., Engeland A., Skurtveit S. Does Pregabalin (Lyrica ®) help patients reduce their use of benzodiazepines? A comparison with gabapentin using the norwegian prescription database. Basic & Clinical Pharmacology & Toxicology. 2010;107(5):883–886. doi: 10.1111/j.1742-7843.2010.00590.x. [PubMed] [CrossRef] [Google Scholar]
16. Markota M., Rummans T. A., Bostwick J. M., Lapid M. I. Benzodiazepine Use in Older Adults: Dangers, Management, and Alternative Therapies. Mayo Clinic Proceedings. 2016;91(11):1632–1639. doi: 10.1016/j.mayocp.2016.07.024. [PubMed] [CrossRef] [Google Scholar]
17. Mersfelder T. L., Nichols W. H. Gabapentin: Abuse, Dependence, and Withdrawal. Annals of Pharmacotherapy. 2016;50(3):229–233. doi: 10.1177/1060028015620800. [PubMed] [CrossRef] [Google Scholar]
doi: 10.1177/1060028015620800. [PubMed] [CrossRef] [Google Scholar]
18. Victorri-Vigneau C., Guerlais M., Jolliet P. Abuse, dependency and withdrawal with gabapentin: A first case report. Pharmacopsychiatry. 2007;40(1):43–44. doi: 10.1055/s-2006-958522. [PubMed] [CrossRef] [Google Scholar]
19. Kruszewski S. P., Paczynski R. P., Kahn D. A. Gabapentin-induced delirium and dependence. Journal of Psychiatric Practice. 2009;15(4):314–316. doi: 10.1097/01.pra.0000358318.73684.df. [PubMed] [CrossRef] [Google Scholar]
20. Reccoppa L., Malcolm R., Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. American Journal on Addictions. 2004;13(3):321–323. doi: 10.1080/10550490490460300. [PubMed] [CrossRef] [Google Scholar]
21. Buttram M. E., Kurtz S. P., Dart R. C., Margolin Z. R. Law enforcement-derived data on gabapentin diversion and misuse, 2002–2015: diversion rates and qualitative research findings. Pharmacoepidemiology and Drug Safety. 2017;26(9):1083–1086. doi: 10.1002/pds.4230. [PubMed] [CrossRef] [Google Scholar]
2017;26(9):1083–1086. doi: 10.1002/pds.4230. [PubMed] [CrossRef] [Google Scholar]
Articles from Case Reports in Psychiatry are provided here courtesy of Hindawi Limited
Using Gabapentin for Anxiety and Depression
Did you know that Gabapentin might be helpful for depression and anxiety?
Gabapentin is an anticonvulsant drug that also goes by Neurontin, Gralise, or Gaborone. It’s initial purpose was to control certain types of seizures in people who have epilepsy, relieving nerve pain from shingles, or calming restless leg syndrome.
Gabapentin has also been used off-label as a treatment for anxiety disorders. It’s possible that your psychiatric provider may prescribe gabapentin to help alleviate some of the symptoms associated with these mental health disorders. Additionally, there is some evidence to suggest that gabapentin can be helpful for individuals who struggle with alcohol use disorders or alcohol dependence.
Let’s take a look at how gabapentin works and why you may be prescribed this medication for anxiety.
Want to speak 1:1 with an expert about your anxiety & depression?
Start with a free assessment
How does Gabapentin work?
Gabapentin is a synthetic version of the neurotransmitter GABA, which means it mimics the role GABA plays in the body. Neurotransmitters are chemicals that carry signals between nerve cells.
The GABA neurotransmitter can help slow down neurons firing in the brain. Gabapentin works in a similar way; it can help quiet the brain and decrease pain transmission in your nerves.
For seizures, gabapentin works by decreasing abnormal excitement in the brain. It can also change the way that the body processes pain, which can help alleviate pain caused by shingles.
While studies don’t typically show effectiveness for improving symptoms of depression, there is evidence that gabapentin may have some benefit for anxiety disorders. A rat study found that gabapentin produced behavioral changes suggestive of anxiolysis, or feelings of calmness.
A rat study found that gabapentin produced behavioral changes suggestive of anxiolysis, or feelings of calmness.
Additionally, a case study of one individual found a clear inverse relationship between dosage of gabapentin and anxiety. The individual — who did not respond well to traditional antidepressant medications such as SSRIs — reported that her anxiety levels were very low on days when she took gabapentin.
So while there is some evidence that gabapentin can be used as a novel medication to treat anxiety and depression, there is not enough research to explain its therapeutic mechanisms.
Even so, your provider may determine that Gabapentin for anxiety or depression is worth prescribing. If so, here are some things you should know.
How to take Gabapentin for anxiety
If you are prescribed gabapentin for anxiety, you should follow the exact dosing prescribed by your provider — the dosing information provided below is for educational purposes only.
Gabapentin can come in a capsule, tablet, or oral solution, but you’ll likely be prescribed capsules to help with anxiety. It is usually taken with a full glass of water, with or without food.
It is usually taken with a full glass of water, with or without food.
Antacids should not be taken within two hours before or after using gabapentin. Antacids can make it harder for your body to absorb gabapentin, making it less effective.
Potential side effects of Gabapentin
As with any medication, side effects may occur when taking gabapentin for anxiety. The main side effects include:
- Dizziness
- Fatigue
- Agitation
- Changes in libido, or sex drive
- Tremors
- Unsteadiness
- Nystagmus (involuntary eye movement)
- Blurred vision
Some of these side effects may resolve after continued use, but if they become severe or long term, reach out to your psychiatric provider immediately.
Gabapentin prescriptions for anxiety
Since gabapentin is a prescription medication, you’ll need to get a prescription from a doctor or psychiatric provider — only after you’ve demonstrated symptoms of anxiety and have been screened for gabapentin allergies. Additionally, you may not be a candidate if you take other medications that have contraindications to gabapentin.
Additionally, you may not be a candidate if you take other medications that have contraindications to gabapentin.
Your provider will provide specific dosing instructions, including a guideline on how frequently you should take gabapentin for your anxiety. As Psycom explains, your dose may start small and increase over time. For anxiety, the dosage of gabapentin will often start at 300 mg once in the evening. The dose can then be increased every three to five days. Some people take 600 mg/day, others take 3,600 mg/day, the maximum dose approved by the FDA.
According to Psycom, gabapentin for depression may follow a different dosage pattern. Dosage of between 900 and 2,000 mg a day works as a mood stabilizer or antidepressant. Some people experience improvement within a week after treatment initiation, others need more time to feel significant symptom relief.
The difference in clinical results means it’s a little tricky to determine how quickly gabapentin might work to help your anxiety.
At Brightside, we use gabapentin alongside other medicines to treat:
- Generalized anxiety disorder
- Hard-to-treat depression
- Insomnia
- Nerve pain
- Panic disorder
- Social anxiety
Adding Gabapentin into your anxiety treatment plan
For anxiety or depression, Gabapentin is typically prescribed alongside other treatment options — here are the most common:
Selective Serotonin Reuptake Inhibitors (SSRI)
SSRI medications are commonly prescribed alongside gabapentin for depression and anxiety. SSRIs work by preventing the reabsorption of serotonin into your brain’s neurons, making the neurotransmitter more abundant. Serotonin is a chemical that is thought to regulate mood and anxiety levels.
There are many different types of SSRIs, but some common examples include Zoloft, Prozac, and Lexapro. SSRIs typically cause fewer side effects than many other types of antidepressants, which is one of the reasons why they’re so popular.
Talk Therapy
Therapy is widely delivered to treat anxiety and depression for those prescribed gabapentin, and it’s highly effective. Although there are many types of depression and anxiety therapy, Cognitive Behavioral Therapy (CBT) is particularly effective. Goal-oriented and structured, CBT addresses the source of irrational thought to alter unfavorable behaviors.
In fact, the most effective way to care for your mental health is to leverage a combination of medication and talk therapy — which enables a 60% better chance of recovery than just one treatment alone.
In conclusion
Gabapentin is an anticonvulsant medication that is prescribed for treating seizures and nerve pain associated with shingles. However, it also is known for producing anti-anxiolytic (anti-anxiety) effects, which is why gabapentin is often prescribed for treating anxiety.
Gabapentin is usually prescribed at a low dose to start but can be gradually increased as needed. While a psychiatric provider will likely not prescribe gabapentin for anxiety or depression as a first course of action, it may be used alongside other treatments to help improve symptoms.
A Brightside psychiatric provider can help you determine what treatment plan is right for you. We offer therapy and psychiatric care right from the comfort of home — including unlimited messaging with your provider and medications delivered right to your door.
Fill out your complimentary assessment to see how Brightside can help you find the right care plan.
Please note: Prescribing decisions are always at the discretion of the provider who abides by your state’s regulations and restrictions. Brightside’s providers can’t prescribe Gabapentin in the following states: Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, or West Virginia.
Sources:
Gabapentin Treatment for Alcohol Dependence: A Randomized Controlled Trial | NCBI
Gabapentin | MedlinePlus
The antiepileptic agent gabapentin (Neurontin) possesses anxiolytic-like and antinociceptive actions that are reversed by D-serine | National Library of Medicine
Treatment of Generalized Anxiety Disorder with gabapentin | NCBI
Gabapentin | Michigan Medicine
Serotonin | Hormone Health Network
Selective serotonin reuptake inhibitors (SSRIs) | Mayo Clinic
Gabapentin Therapy in Psychiatric Disorders: A Systematic Review | NCBI
Gabapentin and pregabalin for bipolar disorder, anxiety and insomnia
Gabapentin is licensed in the US for the treatment of focal seizures and postherpetic neuralgia and in the UK for the treatment of focal seizures and neuropathic pain. Pregabalin has a similar indication and is also indicated for fibromyalgia in the US and generalized anxiety disorder (GAD) in the UK.
Pregabalin has a similar indication and is also indicated for fibromyalgia in the US and generalized anxiety disorder (GAD) in the UK.
Put into practice at 1993, these drugs have become one of the most frequently prescribed drugs, with a significant proportion of prescriptions being off-label prescriptions. A 2002 American study [1] showed that 95% of gabapentin prescriptions are for off-label indications, in at least 10% of psychiatric cases. In the UK, at least half of all prescriptions for gabapentinoids are off-label, and one in five is co-prescribed with opioids. A 2021 study using the American electronic health record network Trinetx [2] showed that gabapentin was prescribed at least once in 13.6% of patients with bipolar disorder (BDD), 11.5% of patients with anxiety disorders, and 12.7 % of patients with insomnia; for pregabalin, these figures were 2.9%, 2.6%, 3.0% respectively.
Despite the widespread use of off-label gabapentin in bipolar disorder, the question of its effectiveness remains unclear. A 2011 meta-analysis of pharmacological treatments for acute mania found no superiority of gabapentin over placebo [3]. Systematic reviews of gabapentin treatment of psychiatric and/or substance use disorders have not shown conclusive evidence for its efficacy in bipolar disorder, but have identified possible efficacy in the treatment of anxiety disorders. nine0003
A 2011 meta-analysis of pharmacological treatments for acute mania found no superiority of gabapentin over placebo [3]. Systematic reviews of gabapentin treatment of psychiatric and/or substance use disorders have not shown conclusive evidence for its efficacy in bipolar disorder, but have identified possible efficacy in the treatment of anxiety disorders. nine0003
In addition to its licensed use for GAD in the UK, pregabalin is used to treat other anxiety disorders and acute anxiety conditions such as preoperative anxiety. The effectiveness of pregabalin for GAD has been established, but its effectiveness is unclear for other anxiety disorders such as social anxiety disorder. Gabapentinoids may also be useful in the treatment of insomnia, but again, firm evidence is lacking.
Overall, studies show that there is only limited evidence for the effectiveness of gabapentinoids in the disorders they are used to treat. Moreover, these drugs have potential risks, including a number of serious side effects, as well as abuse and dependence, which led to their reclassification as controlled drugs in the UK in 2019. In the US, pregabalin (but not gabapentin) has since is a federally controlled drug due to the risk of dependence and abuse. nine0003
In the US, pregabalin (but not gabapentin) has since is a federally controlled drug due to the risk of dependence and abuse. nine0003
A priori, due to their mode of action, gabapentinoids are attractive candidate molecules for use in psychiatry. They act primarily by inhibiting the activity of voltage-gated calcium channels (VGCCs) by binding to the α2δ accessory subunit, which regulates channel traffic and function. Although the genes encoding the α2δ subunits have not yet been associated with psychiatric phenotypes, other VGCC genes, in particular CACNA1C, which encodes a protein of the same name, show strong transdiagnostic associations with various disorders, including bipolar disorder. Administration of calcium channel blockers, which block the α-1 subunit of L-type VGCC, is associated with a reduction in hospital admissions in patients with severe mental illness. Cellular calcium dysregulation has been observed in patients with bipolar disorder and some other psychiatric disorders. nine0003
nine0003
Given the potential risks of gabapentinoids and their widespread off-label use, it is important to carefully assess the evidence base for their use. This systematic review and meta-analysis focuses on the efficacy and tolerability of gabapentin and pregabalin in the treatment of bipolar disorder, anxiety and insomnia.
Published studies were searched in the Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, MEDLINE, MEDLINE In-Process and PsycINFO in the time range from their inception to August 4, 2020. Unpublished studies were searched in international research registries ( ClinicalTrials.gov; ICTRP) and regulatory websites. nine0003
Of the 4268 studies initially identified, 70 studies were included in the review: 55 double-blind RCTs and 15 open-label studies. The risk of bias was unclear for most studies; in a significant proportion of studies, there was an increased risk of error due to dropout.
Four double-blind RCTs were found investigating the efficacy of gabapentin in bipolar disorder. 101 patients were randomized to receive gabapentin, 81 to placebo, 30 to lamotrigine, and 19- carbamazepine. The mean age of all randomized patients was 37.5 years, 64.1% were women.
101 patients were randomized to receive gabapentin, 81 to placebo, 30 to lamotrigine, and 19- carbamazepine. The mean age of all randomized patients was 37.5 years, 64.1% were women.
Three studies evaluated gabapentin in the treatment of acute bipolar disorder in heterogeneous groups of patients with manic/hypomanic, depressive and/or mixed symptoms. In each study, the efficacy of gabapentin was assessed using different outcome measures.
Gabapentin was significantly more effective than lamotrigine and carbamazepine in reducing depressive symptoms on the MMPI-2 depression subscale (50%, 33.5% and 13.6% reductions, respectively), but no difference in improvement on the MMPI mania subscale -2 was not observed. Based on the Global Clinical Impression Scale (CGI-BP), treatment response rates of 50% for lamotrigine, 33% for gabapentin, and 18% for placebo were observed in the clinical global bipolar version in the first phase of the study, but without clear statistical significance. nine0003
nine0003
In a study investigating the addition of gabapentin to the main treatment, a significantly greater improvement in the total score on the YMRS Mania Rating Scale in the placebo group was reported, with no significant difference between groups on the Hamilton Rating Scale for Depression (HAM-D).
One long-term (1-year treatment) study of 25 bipolar patients in clinical remission (13 on gabapentin, 12 on placebo) showed a significant benefit of gabapentin versus placebo in terms of CGI. nine0003
Forty-two double-blind RCTs investigating anxiety disorders/conditions (GAD; social anxiety disorder; preoperative anxiety; PTSD; OCD; panic disorder) were selected. 3539 patients were randomized to receive pregabalin, 525 to gabapentin, 2280 to placebo and 937 to active comparators. The mean age of all randomized patients was 43 years, 60.4% were women. Data were analyzed on 5190 patients from eligible anxiety studies. nine0003
Gabapentinoids are more effective than placebo for a range of disorders on the anxiety spectrum.
Pregabalin is more effective than placebo in the treatment of GAD (SMD -0.37; 95% CI -0.45 to -0.29). The relative risk of withdrawal from the study for all reasons was comparable for pregabalin and placebo. Compared with placebo, pregabalin significantly reduced the relative risk of discontinuation due to failure (RR 0.44; 95% CI 0.28–0.70), but showed a trend towards an increased relative risk of discontinuation due to adverse events (RR 1, 30, 95% CI 0.99 - 1.71).
Pregabalin is more effective than placebo in treating exacerbations of social anxiety (SMD -0.25; 95% CI -0.45 to -0.04). There was no significant difference in the relative risk of dropping out of the study for all reasons or because of adverse events. Compared with placebo, pregabalin showed a trend towards a lower relative risk of dropout due to failure (RR 0.39; 95% CI 0.15 - 1.03).
A 14-week study of gabapentin in social anxiety disorder showed improvement over placebo on several rating scales. A 26-week study investigating pregabalin for relapse prevention found that a fixed dose of 450 mg/day reduced the overall relapse rate compared with placebo (27.5% vs. 43.8%, based on CGI criteria).
A 26-week study investigating pregabalin for relapse prevention found that a fixed dose of 450 mg/day reduced the overall relapse rate compared with placebo (27.5% vs. 43.8%, based on CGI criteria).
Compared to placebo, both pregabalin (SMD -0.55; 95% CI -0.92 to -0.18) and gabapentin (SMD -0.92; 95% CI -1.32 - -0.52) are more effective in reducing preoperative anxiety. Due to the heterogeneity of the studies, a subgroup analysis was performed based on empirically determined threshold doses of dose-response efficacy.
High doses (>600 mg) of gabapentin are effective in reducing preoperative anxiety (SMD -1.30; 95% CI -1.72 to -0.87), while low doses (600 mg) are ineffective. Due to the high degree of heterogeneity among studies in the high dose group, the validity of the estimate of the effect of high doses remains uncertain. Visual inspection of the gabapentin vs. placebo graph suggests possible effects from small trials, with larger effect sizes seen in high-dose gabapentin trials with smaller sample sizes. There was no significant difference between the low (≤150 mg) and high (300 mg) pregabalin dose subgroups. Interestingly, low dose pregabalin was more effective than placebo (SMD -0.29; 95% CI -0.54 - -0.05), while the high dose does not.
There was no significant difference between the low (≤150 mg) and high (300 mg) pregabalin dose subgroups. Interestingly, low dose pregabalin was more effective than placebo (SMD -0.29; 95% CI -0.54 - -0.05), while the high dose does not.
It is not possible to conduct a meta-analysis of the tolerability of pregabalin and gabapentin in studies of preoperative anxiety. The short evaluation time (lasting hours rather than weeks) means that dropouts have occurred in very few studies. In such cases, dropout was due to the practicalities of the preoperative procedures rather than the decision of the participants.
Three double-blind RCTs were included in the review, two of which evaluated the addition of pregabalin to the treatment of PTSD and OCD, and one evaluated the effect of gabapentin in the treatment of panic disorder. Pregabalin supplementation is more effective than placebo in chronic post-combat PTSD and in OCD refractory to SSRIs. However, eight weeks of flex-dose gabapentin is no more effective than placebo in patients with panic disorder. nine0003
nine0003
Eight double-blind RCTs evaluated the efficacy of gabapentin in sleep disorders. In two studies, 111 patients were randomized to gabapentin and 60 to placebo (mean age 44.8 years; 44.9% women). In participants with alcohol dependence and related sleep disorders, gabapentin did not show improvement in sleep scores compared to placebo. The relative risk of withdrawal from the study for all reasons was comparable for gabapentin and placebo. nine0003
Only one double-blind RCT evaluated the efficacy of pregabalin (n=121) versus venlafaxine (n=125) and placebo (n=128) in the treatment of sleep disorders (mean age of all randomized patients 40.8 years, 60.7% women). Pregabalin significantly reduced sleep problems scores compared to placebo at weeks 4 and 8.
Compared to previous studies, this systematic review covers more disorders and databases, considers only double-blind RCTs to summarize evidence of efficacy, and includes unpublished data. nine0003
nine0003
Review shows minimal evidence to support the use of gabapentinoids for bipolar disorder and insomnia.
Moderate effect size observed across the spectrum of anxiety disorders; a significant effect is observed in certain anxiety states; The effect of gabapentin on preoperative anxiety is dose dependent.
With regard to BAD, the small number of double-blinded RCTs investigating gabapentin and the lack of studies on pregabalin do not support evidence of efficacy of gabapentinoids. Quantitative synthesis was not performed due to the heterogeneity of the study population, study design, and outcome scoring systems. The evidence collected is inconclusive and does not justify the use of gabapentinoids in the treatment of bipolar disorder. nine0003
An analysis of anxiety-related studies showed a statistically significant effect size in favor of gabapentinoids across the spectrum of anxiety disorders/conditions. This transdiagnostic effect is explained by the fact that anxiety, anxiety proneness, and pathological anxiety are all mediated by the same neural network and common phenotype seen in anxiety disorders.
There is a dose-dependent effect of gabapentin on preoperative anxiety, with >600 mg required to treat acute anxiety. The analysis was performed post-hoc when examining significant study heterogeneity, and its findings should be used to develop hypotheses for future studies. The choice of dose cut-offs was based on empirical reports of dose-dependent effects in GAD, which is a limitation of this subgroup analysis. nine0003
In GAD, remission/moderate anxiety was observed at total daily doses of gabapentin ≥900 mg/day, recurrent severe anxiety at <600 mg/day. A similar approach was used for pregabalin, based on reports of differences in efficacy between 150 mg/day and 200–600 mg/day for GAD.
A meta-analysis of studies on alcohol-related insomnia found that gabapentin was no better than placebo. Gabapentinoids may improve sleep in patients with a range of clinical conditions, such as insomnia associated with GAD and neuropathic pain, but the extent to which direct or indirect effects influence sleep parameters in these conditions is unclear. While the studies of pregabalin for GAD included in this review reported improvements in HAM-A sleep, they were not included in the meta-analysis due to doubts about the validity of the HAM-A subscales for insomnia. nine0003
While the studies of pregabalin for GAD included in this review reported improvements in HAM-A sleep, they were not included in the meta-analysis due to doubts about the validity of the HAM-A subscales for insomnia. nine0003
On the one hand, there is a limited nature of evidence for the effectiveness of gabapentinoids, on the other hand, there is strong evidence for their side effects and potential harm. Gabapentinoids commonly cause central nervous system depressant side effects, such as drowsiness and dizziness, which increase the risk of accidental physical injury and traffic accidents. There is also growing concern about the addictive potential and harms of gabapentinoids when used with opioids. nine0003
An important weakness of the evidence is its methodological quality and the risk of bias in the studies. The prominent side effects of gabapentinoids make it difficult to blind study participants, possibly leading to an overestimation of effect sizes. Therefore, the results of studies indicating the effectiveness of gabapentinoids should be treated with caution, especially when such results justify their off-label use. Against this background, the widespread use of off-label gabapentinoids seems unreasonable and requires more careful study. nine0003
Against this background, the widespread use of off-label gabapentinoids seems unreasonable and requires more careful study. nine0003
Neurobiological and pharmacological considerations supporting the choice of drug target should never take precedence over empirical clinical evidence of efficacy and safety. However, they may be useful for further research.
The role of VGCC in psychiatric disorders is supported by genomic data. The strongest evidence is for schizophrenia and bipolar disorder, with associations with VGCC seen in a range of psychiatric disorders (albeit not yet found in anxiety disorders). Genetic associations with VGCC are mainly associated with α-1 and β subunits; but we do not have reliable information about the genes of the α2δ subunits. nine0003
In addition, the desired nature and direction of manipulation required to achieve therapeutic benefit remains to be determined. While the current understanding of VGCC is based on channel blocking, alternative and more subtle approaches are likely to be useful as well.
For example, the VGCC genes encode several isoforms with different properties, including sensitivity to existing channel blockers, and with varying tissue expression. The impact of rare mutations also points to the need to modulate rather than simply block VGCC function in psychiatric disorders. In the case of CACNA1C, gain-of-function mutations cause Timothy Syndrome, which is characterized by autism, but autism is also known to occur with loss-of-function mutations. All of this highlights the need for therapeutic agents capable of fine-tuning function, perhaps by targeting specific isoforms or through homeostatic action, which should ultimately maximize clinical benefit and minimize side effects. nine0003
Targeting the α2δ subunits characteristic of gabapentinoids may lead to subtle modulation of VGCC function. α2δ subunits increase the density of VGCC on the plasma membrane, direct the traffic of these channels to subcellular sites, and enhance function by changing their biophysical properties. Gabapentin reduces the number of α2δ and α-1 subunits on the cell surface and attenuates VGCC activity, indicating its inhibitory role. However, the exact effect of gabapentin on calcium channels depends on the stoichiometry of the VGCC accessory subunits, which, like other VGCC subunits, vary in number in different tissues, due to which gabapentinoids may have different effects on VGCC in different cell types. nine0003
Gabapentin reduces the number of α2δ and α-1 subunits on the cell surface and attenuates VGCC activity, indicating its inhibitory role. However, the exact effect of gabapentin on calcium channels depends on the stoichiometry of the VGCC accessory subunits, which, like other VGCC subunits, vary in number in different tissues, due to which gabapentinoids may have different effects on VGCC in different cell types. nine0003
It is possible that the effect of gabapentinoids on anxiety is mediated by α2δ-dependent but VGCC-independent mechanisms. Notably, α2δ-1 interacts with NMDA receptors (NMDARs), promoting dendritic spine maturation and NMDAR traffic.
Thus, it is interesting not only to elucidate the clinical effect of gabapentinoids, but also to establish their molecular mechanisms of action in order to understand the pathophysiology and identify new therapeutic strategies. A strategy for personalized medicine should take into account genomic and other factors influencing VGCC function and psychiatric disorders. nine0003
nine0003
Gabapentinoids are generally effective across the spectrum of anxiety disorders, and it is likely that this is due to pharmacological effects on transdiagnostic anxiety phenotypes mediated by α2δ-dependent mechanisms. Anxiety is the most common comorbid pathology in patients with bipolar disorder [4], which partly reflects a common genetic predisposition [5]. Comorbid anxiety is associated with greater symptom severity and worse clinical outcomes. To date, there are no clinical studies on the effectiveness of gabapentinoids in the treatment of anxiety in bipolar disorder. The development of treatments for “bipolar anxiety” using gabapentinoids or modified α2δ ligands may become a promising area of research in the future. nine0003
Systematic review and meta-analysis show that widespread psychiatric prescription of off-label gabapentinoids is not supported by reliable evidence, except for certain anxiety conditions. Thus, despite the attractive genetic and pharmacological rationale for their use, caution should be exercised until evidence of efficacy and safety is found. Presumably, it is possible to develop modified α2δ ligands that target specific subtypes or isoforms with a more favorable therapeutic profile. nine0003
Presumably, it is possible to develop modified α2δ ligands that target specific subtypes or isoforms with a more favorable therapeutic profile. nine0003
Translated by: Filippov D.S.
Source: Hong, J.S.W., Atkinson, L.Z., Al-Juffali, N. et al. Gabapentin and pregabalin in bipolar disorder, anxiety states, and insomnia: Systematic review, meta-analysis, and rationale. Mol Psychiatry (2021).
References:
[1] Hamer AM, Haxby DG, McFarland BH, Ketchum K. Gabapentin use in a managed Medicaid population. J Manag Care Spec Pharm. 2002;8:266–71. nine0003
[2] Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236,379 COVID-19 survivors: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–27.
[3] Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet 2011;378:1306–15.
Lancet 2011;378:1306–15.
[4] Spoorthy MS, Chakrabarti S, Grover S. Comorbidity of bipolar and anxiety disorders: an overview of trends in research. World J Psychiatry. 2019;9:7–29.
[5] Lopes FL, Zhu K, Purves KL, Song C, Ahn K, Hou L, et al. Polygenic risk for anxiety influences anxiety comorbidity and suicidal behavior in bipolar disorder. Transl Psychiatry. 2020;10:298.
The use of gabapentin in the treatment of children with cancer
Pain relief
Trademarks:
Neurontin®, Gralise®
Often used for:
Convulsions, migraines and neuralgia
Gabapentin is a drug that helps relieve seizures. This drug also helps relieve pain in peripheral neuropathy and other neuralgia.
The dosage of gabapentin is increased gradually until the required level is reached. Gabapentin begins to work within a few days.
This drug is available in regular and extended release forms. Follow dosage instructions carefully. nine0003
Follow dosage instructions carefully. nine0003
Oral tablet or capsule form
Oral liquid form
- Drowsiness
- Increased fatigue or general weakness
- Nausea and vomiting
- Dizziness
- Diarrhea
- Constipation
- Rash
- Abdominal pain
- Nervous tic of the eye
The listed side effects are not observed in all patients who are prescribed gabapentin. The most common side effects are highlighted in bold, but others are not excluded. Report all possible side effects to your doctor or pharmacist.
Be sure to discuss these and other recommendations with your doctor or pharmacist.
- This drug may cause dizziness and drowsiness and increase the risk of falls. nine0266
- Stop taking gabapentin only with your doctor's advice.
- Patients of childbearing age who have been prescribed gabapentin should consult their physician before planning pregnancy.