Aricept for adhd
An open trial of adjunctive donepezil in attention-deficit/hyperactivity disorder
Comparative Study
. 2005 Dec;15(6):947-55.
doi: 10.1089/cap.2005.15.947.
Timothy E Wilens 1 , James Waxmonsky, Megan Scott, Allison Swezey, Anne Kwon, Thomas J Spencer, Joseph Biederman
Affiliations
Affiliation
- 1 Pediatric Psychopharmacology Unit, YAW 6A, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114, USA. [email protected]
- PMID: 16379515
- DOI: 10.1089/cap.
2005.15.947
Comparative Study
Timothy E Wilens et al. J Child Adolesc Psychopharmacol. 2005 Dec.
. 2005 Dec;15(6):947-55.
doi: 10.1089/cap.2005.15.947.
Authors
Timothy E Wilens 1 , James Waxmonsky, Megan Scott, Allison Swezey, Anne Kwon, Thomas J Spencer, Joseph Biederman
Affiliation
- 1 Pediatric Psychopharmacology Unit, YAW 6A, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114, USA. [email protected]
- PMID: 16379515
- DOI: 10.
 1089/cap.2005.15.947
1089/cap.2005.15.947
Abstract
Objective: Despite available pharmacotherapeutics, a number of youths with attentiondeficit/ hyperactivity disorder (ADHD) continue to experience residual symptoms and prominent executive function (EF) deficits resulting in impairment in multiple domains. We sought to determine if donepezil, used adjunctively to stimulant medication, would improve residual symptoms of ADHD and EF deficits.
Methods: In a 12-week open trial, we treated 7 children and 6 adults who had ADHD and evidence of further EF deficits with adjunctive donepezil. All subjects were stabilized on stimulants, at which time donepezil was initiated at 2.5 mg daily and increased to a maximum of 10 mg over the 12-week trial.
Results: Of 13 subjects receiving medication, 7 completed the trial. There was no clinically or statistically significant improvement in the ADHD Rating Scale and the Executive Function Checklist, our primary outcome measures. A majority of individuals experienced nonserious adverse events.
There was no clinically or statistically significant improvement in the ADHD Rating Scale and the Executive Function Checklist, our primary outcome measures. A majority of individuals experienced nonserious adverse events.
Conclusions: Results of this small open study suggest that donepezil augmentation of stimulants is not well tolerated and does not appear useful for the treatment of residual ADHD and/or EF deficits.
Similar articles
-
Donepezil for Tourette's disorder and ADHD.
Hoopes SP. Hoopes SP. J Clin Psychopharmacol. 1999 Aug;19(4):381-2. doi: 10.1097/00004714-199908000-00019. J Clin Psychopharmacol. 1999. PMID: 10440471 No abstract available.
-
Donepezil for cognitive decline following coronary artery bypass surgery: a pilot randomized controlled trial.
Doraiswamy PM, Babyak MA, Hennig T, Trivedi R, White WD, Mathew JP, Newman MF, Blumenthal JA. Doraiswamy PM, et al. Psychopharmacol Bull. 2007;40(2):54-62. Psychopharmacol Bull. 2007. PMID: 17514186 Clinical Trial.
-
Adjunctive donepezil in attention deficit hyperactivity disorder youth: case series.
Wilens TE, Biederman J, Wong J, Spencer TJ, Prince JB. Wilens TE, et al. J Child Adolesc Psychopharmacol. 2000 Fall;10(3):217-22. doi: 10.1089/10445460050167322. J Child Adolesc Psychopharmacol. 2000. PMID: 11052411
-
Attention deficit hyperactivity disorder in adults.
Rösler M, Casas M, Konofal E, Buitelaar J. Rösler M, et al. World J Biol Psychiatry. 2010 Aug;11(5):684-98. doi: 10.
 3109/15622975.2010.483249. World J Biol Psychiatry. 2010. PMID: 20521876 Review.
3109/15622975.2010.483249. World J Biol Psychiatry. 2010. PMID: 20521876 Review. -
Relevance of donepezil in enhancing learning and memory in special populations: a review of the literature.
Yoo JH, Valdovinos MG, Williams DC. Yoo JH, et al. J Autism Dev Disord. 2007 Nov;37(10):1883-901. doi: 10.1007/s10803-006-0322-8. Epub 2007 Jan 13. J Autism Dev Disord. 2007. PMID: 17221321 Review.
See all similar articles
Cited by
-
Preclinical Evaluation of Attention and Impulsivity Relevant to Determining ADHD Mechanisms and Treatments.
Kenton JA, Young JW. Kenton JA, et al. Curr Top Behav Neurosci. 2022;57:291-320. doi: 10.1007/7854_2022_340. Curr Top Behav Neurosci.
 2022. PMID: 35606639
2022. PMID: 35606639 -
Functional Neuroimaging in Psychiatry-Aiding in Diagnosis and Guiding Treatment. What the American Psychiatric Association Does Not Know.
Henderson TA, van Lierop MJ, McLean M, Uszler JM, Thornton JF, Siow YH, Pavel DG, Cardaci J, Cohen P. Henderson TA, et al. Front Psychiatry. 2020 Apr 15;11:276. doi: 10.3389/fpsyt.2020.00276. eCollection 2020. Front Psychiatry. 2020. PMID: 32351416 Free PMC article. Review.
-
Adult ADHD: Risk Factor for Dementia or Phenotypic Mimic?
Callahan BL, Bierstone D, Stuss DT, Black SE. Callahan BL, et al. Front Aging Neurosci. 2017 Aug 3;9:260. doi: 10.3389/fnagi.2017.00260. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28824421 Free PMC article.

-
Targeting the nicotinic cholinergic system to treat attention-deficit/hyperactivity disorder: rationale and progress to date.
Potter AS, Schaubhut G, Shipman M. Potter AS, et al. CNS Drugs. 2014 Dec;28(12):1103-13. doi: 10.1007/s40263-014-0208-9. CNS Drugs. 2014. PMID: 25349138 Free PMC article. Review.
-
The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function.
Parker MO, Brock AJ, Walton RT, Brennan CH. Parker MO, et al. Front Neural Circuits. 2013 Apr 8;7:63. doi: 10.3389/fncir.2013.00063. eCollection 2013. Front Neural Circuits. 2013. PMID: 23580329 Free PMC article. Review.
See all "Cited by" articles
Publication types
MeSH terms
Substances
Grant support
- DA11929/DA/NIDA NIH HHS/United States
Dementia Drug May Help Improve ADHD Symptoms
ATHENS, Greece — A drug approved for the treatment of dementia appears to improve executive function in adults with attention-deficient/hyperactivity disorder (ADHD) when administered with standard stimulant therapy, preliminary research suggests.
Initial results from the small, randomized controlled trial indicate that adjunctive memantine (Namenda, Forest Laboratories, Inc) with osmotic release oral system-methylphenidate (OROS-MPH) was associated with improvement in ADHD patients with executive function deficits (EFDs).
"Most of the benefits that memantine produces in dementia are improvements in cognition, so we reasoned that if memantine can improve cognition in the context of dementia, it could improve cognition outside the context of dementia," study investigator Joseph Biederman, MD, chief of pediatric psychopharmacology and adult ADHD at the Massachusetts General Hospital, in Boston, told Medscape Medical News.
"What we saw was a trend towards normalization on several measures of executive function, as reflected by changes on the BRIEF scale with memantine compared with placebo," he added.
The study was presented here at the 12th World Congress of Biological Psychiatry.
Wide Range of Difficulties
According to investigators, EFDs produce a wide range of difficulties that affect scholastic achievement in particular as well as the ability to work, plan, organize, and manage time well.
"People can have a very clear response to ADHD treatment in terms of improvement in ADHD symptoms but can still be massively affected with EFD," Dr Biederman said.
"We recently showed that the effect size on executive function is very limited with OROS methylphenidate compared with its very large effect size on ADHD symptoms. So we wanted to see if there was any possibility that a medicine with a different mechanism of action like memantine could have some benefit," he added.
A total of 26 men and women between aged 18 to 57 years with at least moderately severe ADHD and EFDs, defined as two or more abnormal test results out of nine subscales included in the BRIEF- A, were included in the study.
All participants received open-label OROS methylphenidate and were then randomly assigned to receive either memantine 10 mg twice a day or placebo. Patients were evaluated weekly for 6 weeks and then biweekly for up to 12 weeks.
"We used a wide range of ADHD rating scales to assess the effect of augmentation memantine, including the Adult ADHD Investigator Symptom Report, Clinical Global Impression, and the CANTAB cognitive battery," said Dr Biederman, "and there were no significant clinical or demographic differences between the two groups. "
"
Because of the small sample size, investigators considered a standardized mean difference (equivalent to effect size) of ≥0.5 and odds ratios ≥2 as indicators of trend improvements.
Twelve participants received memantine and 14 received placebo.
Memantine had a standardized mean difference >0.5 on both the Behavior Rating Inventory of Executive Functions–Adult Inhibition (P = .27) and the Self-Monitor subscales (P = .40) when compared with patients receiving placebo.
On the other hand, for patients receiving placebo, with regard to organization of materials, the standardized mean difference was >0.5 (P = .66). No significant changes were noted on the Cambridge Neuropsychological Test Automated Battery in either treatment group.
"While nothing was strikingly significant, there was a trend towards more normalization of several measures of executive function with memantine than with placebo," said Dr Biederman.
Cautious Optimism
Commenting on the findings for Medscape Medical News, Jeffrey Newcorn, MD, associate professor of psychiatry and pediatrics, Icahn School of Medicine at Mount Sinai, in New York City, noted that EFDs are a major problem for a subgroup of adults with ADHD.
"These deficits have often been resistant to treatment, so any improvement that can be realized using a different approach than the current standard ADHD treatments would be very good news," he added.
However, Dr Newcorn observed that these are preliminary results obtained in a small sample, so one needs to be cautious in drawing conclusions. Nevertheless, the data seem very promising and certainly should be followed up, he added.
Dr Biederman currently receives research support from the following sources: the Department of Defense, the US Food and Drug Administration, Ironshore, Lundbeck, Magceutics Inc, Merck, PamLab, Pfizer, Shire Pharmaceuticals Inc, SPRITES, Sunovion, Vaya Pharma/Enzymotec, and the National Institutes of Health. He also has a US patent application pending through Massachusetts General Hospital corporate licensing on a method to prevent stimulant abuse. Dr Newcorn has disclosed no relevant financial relationships.
World Congress of Biological Psychiatry. Presented June 15, 2015.
Presented June 15, 2015.
Lundbeck relied on Tourette syndrome
Image: Radachynskyi Serhii/Shutterstock.IMPORTANT, NEWS, M&AModulation of the endocannabinoid system should help.
Danish Lundbeck buys Abide Therapeutics, a neurological therapy company. The deal, expected to close in the second quarter, is $250 million up front, plus a potential $150 million as certain development and commercialization milestones for experimental drug programs are reached.
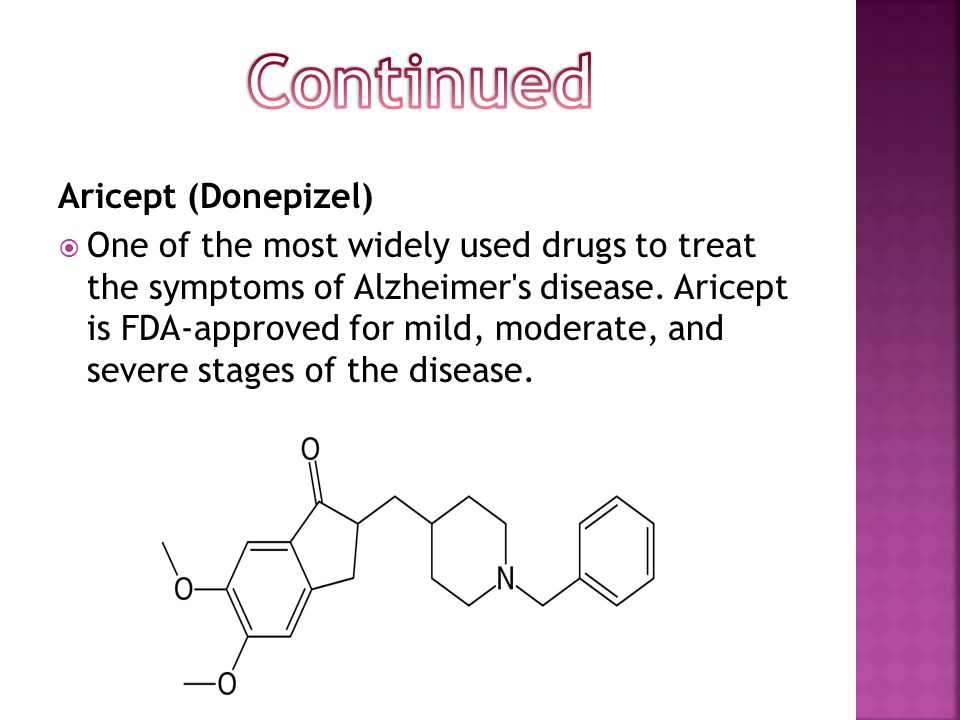
Abide has a chemoproteomic platform for the detection of strong and selective inhibitors of serine hydrolases. This is a vast superfamily of enzymes, comprising 250 diverse enzymes, which, despite a modest 1% presence in the human proteome, play key roles in a variety of physiological and pathophysiological processes, including thrombosis, digestion, neural signaling, inflammation, and oncology. Currently, drugs have been proposed that target a few targets from the serine hydrolase superfamily: for example, Xenical (Xenical, orlistat), Januvia (Januvia, sitagliptin), Aricept (Aricept, donepezil), Xarelto (Xarelto, rivaroxaban) - not counting antibiotics affecting bacterial serine hydrolases.
Currently, drugs have been proposed that target a few targets from the serine hydrolase superfamily: for example, Xenical (Xenical, orlistat), Januvia (Januvia, sitagliptin), Aricept (Aricept, donepezil), Xarelto (Xarelto, rivaroxaban) - not counting antibiotics affecting bacterial serine hydrolases.
Abide's main focus is drug targeting of monoacylglycerol lipase (MGLL, MAGL), which converts 2-arachidonoylglycerol (2-AG), an endogenous cannabinoid receptor (CNR) agonist, into fatty acids and glycerol. Pharmacological inactivation of MGLL leads to an increase in the concentration of 2-AG in the central nervous system (CNS) and a concomitant decrease in arachidonic acid and eicosanoids, which is reflected by antinoceptive (painkillers), anxiolytic (sedative) and anti-inflammatory effects, and without inducing the full range of psychoactive effects provided by direct CNR agonists like exogenous cannabinoids like cannabis. Since the endocannabinoid system is extremely important for maintaining the proper activity of the CNS, its modulation can restore the synaptic balance lost due to pathological processes.
Since the endocannabinoid system is extremely important for maintaining the proper activity of the CNS, its modulation can restore the synaptic balance lost due to pathological processes.
Aybad's leading drug candidate is the MGLL inhibitor ABX-1431, which potentiates the endocannabinoid system by stimulating the cannabinoid type 1 receptor (CB 1 ). The low molecular weight compound is being clinically tested in the treatment of Tourette's syndrome caused by levodopa dyskinesia and neuropathic pain.
When it comes to Tourette's syndrome, the industrial pipeline is pathetic and poor, because the disease, like other mental disorders, is very difficult to treat with pharmacotherapy. For example, Ingrezza (valbenazine), a highly selective vesicular monoamine transporter 2 (VMAT2) inhibitor, has already failed clinical trials twice. So Lundbeck, despite the encouraging performance of ABX-1431, will have to prove to investors the justification for spending money on the absorption of Abide: the results of phase IIa clinical trials will not be ready until next year.
Aybad's preclinical development includes ABX-1762 and ABX-1626, other MGLL inhibitors targeting acute CNS conditions and inflammatory diseases, and ABX-1772, which, being a dual inhibitor of MGLL and fatty acid amide hydrolase (FAAH ), is being studied with Celgene in the treatment of epilepsy.
Abide Therapeutics Experimental Conveyor.In general, the prospects for therapeutic CNS coverage of Aybad drug compounds are wide and may be in demand in such problematic neurological and psychiatric pathologies as Parkinson's disease, tardive dyskinesia, Huntington's disease, obsessive-compulsive disorder, agitation, attention deficit hyperactivity disorder (ADHD). ).
Lundbeck needs to update research projects to reflect recent clinical setbacks, when in October 2018 the experimental antipsychotic Lu AF35700, characterized by an increased affinity for the dopamine D 1 receptor, failed in therapy for treatment-resistant schizophrenia, and in February 2019 th, the atypical antipsychotic Rexulti (Brexpiprazole) failed to prove the viability of the treatment of manic episodes in bipolar affective disorder type I.
Mosmedpreparaty
Important information
Mosmedpreparaty.ru is a specialized research and reference and analytical service of the Mosmedpreparaty group of companies, targeted at key events in the global pharmaceutical, biotechnology, medicine and healthcare industries.
- Nothing on Mosmedpreparaty.ru is an advertisement or promotion of medicines, methods of treatment, medical services.
- Information and publications Mosmedpreparaty.ru are for educational and educational purposes only.
- Medical information broadcast by Mosmedpreparaty.ru is intended only for specialists in the field of healthcare and the field of drug circulation.
- The medical information contained on Mosmedpreparaty.ru is not intended to be used as a substitute for consultation with a healthcare professional.
- Nothing on Mosmedpreparaty.ru should be construed as providing medical advice or recommendations and should not form the basis for making any decision or taking any action without the participation of a healthcare professional.

Presence on the web resource Mosmedpreparaty.ru and familiarization with its content means that you have read the "User Agreement" and accepted its terms.
Topics: Abide Therapeutics, Lundbeck, pain, dyskinesia, Tourette syndrome, chronic neuropathic pain, epilepsyBusiness expert of the R&A office Mosmedpreparaty.ru .
Additional information about Alexey and his contact details are available in Section Research Office .
A New Hope for Children with Autism Spectrum Disorders
In a recent study at Sheba Medical Center, researchers found that combining drug treatment with Donepezil with choline (a dietary supplement) resulted in improved receptive language skills in children with autism.
The greatest progress was observed among children aged 5 to 10 years. The same group of scientists conducted another study that showed the extraordinary benefits of working with medical clowns in the treatment of autism.
Dr. Lydia Gabis, head of the Center for Child Development named after. Weinberg at the Children's Hospital. Edmond and Lily Safra, led the Donepezil and Choline study. Donepezil is commonly used to treat Alzheimer's disease and is sold under the brand name Aricept. The study involved 60 children and adolescents with autism spectrum disorders.
At the end of the 9-month study, Dr. Gabis noted a significant improvement in receptive language skills, which refers to the ability to understand words and language. As a rule, in children with autism, this ability is impaired. In the younger group, there was a significant improvement, even 6 months after treatment, while among adolescents, improvements were minimal, including the presence of increased irritability as a side effect. The only additional side effect reported was the minor gastrointestinal disturbances experienced by both groups.
“We are deeply excited about the results of the study among young children.
This is a huge breakthrough showing that the main symptoms of autism can be treated with a combination of pharmacological drugs. This gives hope for further progress, and we look forward to seeing other scientists arrive at the same results, but with larger sample sizes. We are making significant progress in the treatment of autism spectrum disorders and offer hope to families around the world struggling with this disease,” said Dr. Gabis.
Dr. Lydia Gabis, head of the Child Development Center named after. Weinberga
More than 10 years ago, Dr. Gabis founded the Keshet Center, one of the largest autism therapy centers in the world. This state-of-the-art facility practices a personalized, family-centered approach to early diagnosis, intervention, and research for autistic disorders.
Another pioneering study by Sheba at the Keshet Center was to study the effects of contact with medical clowns as part of group therapy. It turned out that clowns have a very positive effect on children with autism spectrum disorder between the ages of two and six.