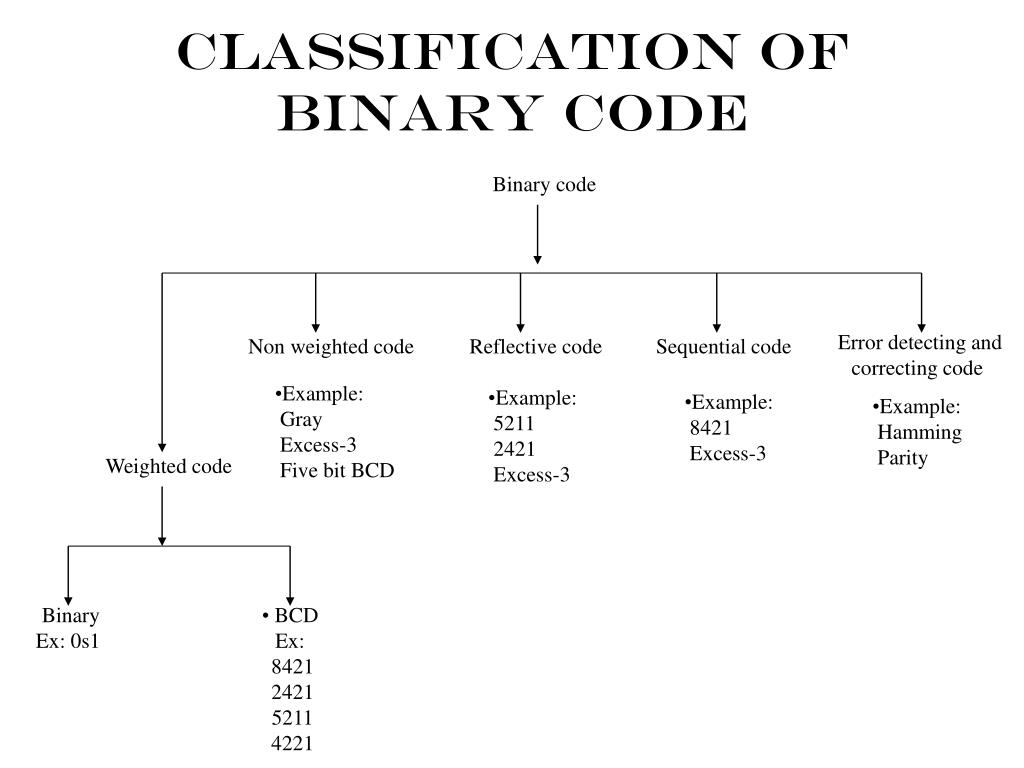
Valacyclovir side effects depression
Common and Serious Side Effects of Valacyclovir
- How does valacyclovir work?
- Does valacyclovir have any side effects?
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
Valacyclovir (brand name Valtrex) is an antiviral medicine that works against viral infections, especially herpes infections.
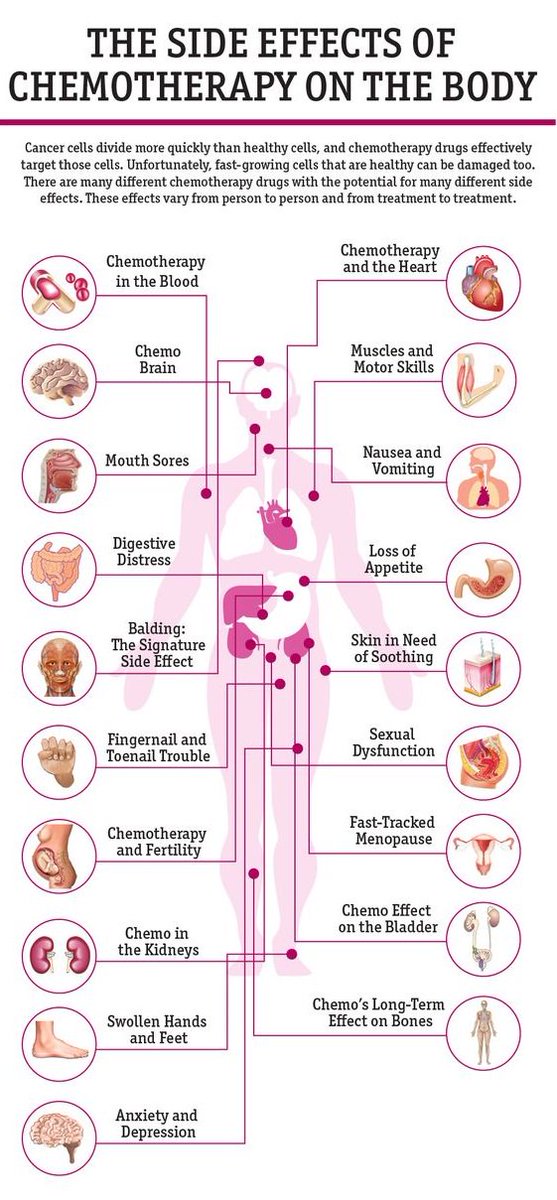
The types of herpes valacyclovir treats include genital herpes, cold sores, chickenpox, and shingles. While valacyclovir is generally well-tolerated, certain groups are at higher risk of more serious side effects. Read on for more about this treatment, its side effects, and who should avoid it.
Effective treatment for cold sores
Peace of mind and the prescription treatment you need, without the uncomfortable trips to the pharmacy.
Learn more
Valacyclovir treats viral infections by preventing the virus from multiplying and spreading throughout the body (Ormrod, 2000). Only available by prescription, valacyclovir is most effective if taken as soon as possible after developing the virus. Unfortunately, valacyclovir does not cure the infection.
Taking valacyclovir can make your herpes symptoms less painful and resolve faster than they would without medication, but it cannot cure you of the virus—which can live and hide in your body. In some cases, valacyclovir is used long-term (suppressive therapy) to prevent or suppress future herpes virus outbreaks.
While valacyclovir is generally well tolerated, some people may experience side effects. The most common side effects of valacyclovir include (UpToDate, n.d.):
- Headache
- Nausea
- Abdominal pain
- Tiredness (fatigue)
- Depression
- Skin rash
Certain groups are at higher risk of more serious side effects, including (UpToDate, n. d.):
d.):
- Elderly individuals: May experience central nervous system side effects like agitation, hallucinations, confusion, seizures, etc.
- People with kidney disease or kidney problems: Since the kidneys remove valacyclovir from the body, people with decreased kidney function need a reduced dose. Otherwise, too much of the drug can accumulate, leading to kidney failure and symptoms like drowsiness, reduced urine output, and swelling of legs or feet (FDA, 2008).
- People with low immune system function: Those with low immune system function, such as people with HIV/AIDS or bone marrow transplants, may develop a medical condition that affects their blood cells like thrombotic thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS).
- Pregnant or breastfeeding women: There are no adequate and well-controlled studies of valacyclovir use in pregnant women. It should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus (FDA, 2008).
 Valacyclovir is considered Pregnancy Category B. Breastfeeding women should use caution and seek medical advice before taking valacyclovir (FDA, 2008).
Valacyclovir is considered Pregnancy Category B. Breastfeeding women should use caution and seek medical advice before taking valacyclovir (FDA, 2008).
Herpes Last updated: Oct 04, 2019 4 min read
Seek medical advice at the first sign of any serious side effects. Anyone with a history of an allergic reaction to valacyclovir or acyclovir should avoid taking it.
Besides the side effects listed, valacyclovir can also interact with other prescription drugs that you may be taking. Cladribine, a chemotherapy medicine used to treat leukemia, loses its effectiveness if taken with valacyclovir. If you take foscarnet (also an antiviral drug) with valacyclovir, it increases the risk of damage to the kidney (UpToDate, n.d.). Avoid taking valacyclovir if you are also taking any of these medications to prevent drug interactions.
Taking valacyclovir at the same time as getting varicella or zoster vaccines may also interfere with the vaccine’s effectiveness (UpToDate, n. d.). Avoid taking valacyclovir 24 hours before and 14 days after getting the vaccines. Always tell your healthcare provider about any medical problems you may have or drugs that you are taking before starting valacyclovir.
d.). Avoid taking valacyclovir 24 hours before and 14 days after getting the vaccines. Always tell your healthcare provider about any medical problems you may have or drugs that you are taking before starting valacyclovir.
- Food and Drug Administration (FDA) – Valacyclovir (2008). Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020487s014lbl.pdf on July 15, 2020
- Ormrod, D., & Goa, K. (2000). Valaciclovir: a review of its use in the management of herpes zoster. Drugs, 59(6), 1317–1340. doi: 10.2165/00003495-200059060-00009. Retrieved from https://link.springer.com/article/10.2165/00003495-200059060-00009
- UpToDate – Valacyclovir: Drug Information (n.d.). Retrieved on July 15, 2020 from https://www.uptodate.com/contents/valacyclovir-drug-information?topicRef=8293&source=see_link
Side Effects, Dosage, Uses, and More
Highlights for valacyclovir
- Valacyclovir oral tablet is available as a brand-name drug and a generic drug.
 Brand name: Valtrex.
Brand name: Valtrex. - Valacyclovir comes only as a tablet you take by mouth.
- Valacyclovir oral tablet is used to treat viral infections caused by a group of viruses called herpes simplex viruses. It’s used to treat cold sores (oral herpes), shingles, or chickenpox. It’s also used to treat or prevent flare-ups of genital herpes.
- Blood disorders warning: For certain people, this drug can cause thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS). These conditions cause a severely low level of red blood cells and platelets in your body. TTP or HUS can result in death. You’re at risk of these problems if you’ve had a bone marrow or a kidney transplant. You’re also at risk if you have advanced HIV or AIDS.
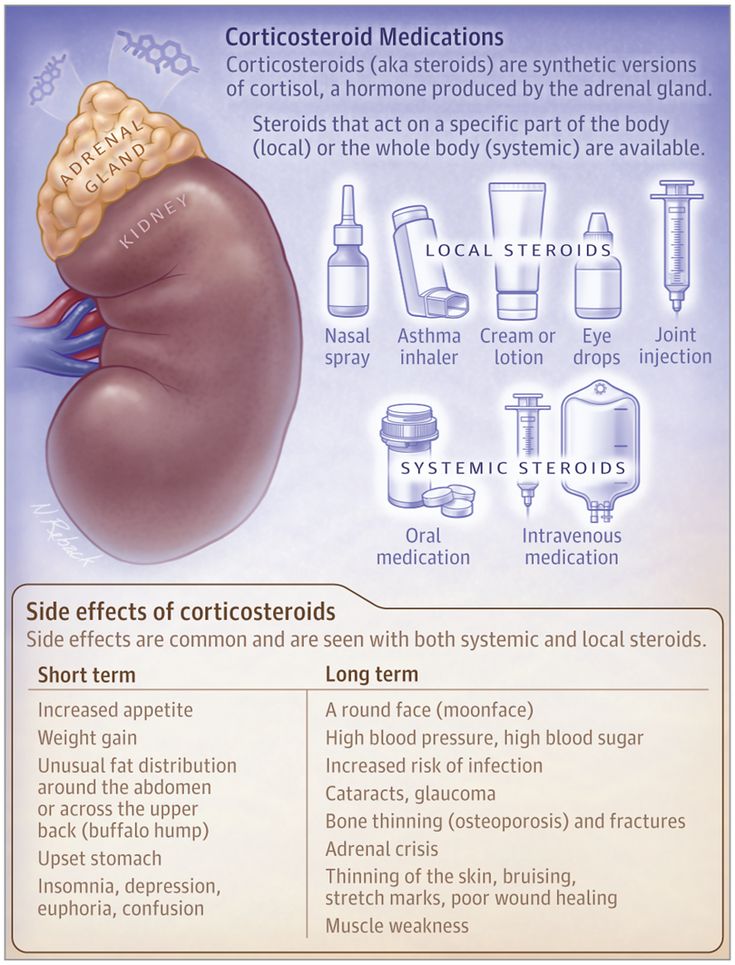
- Kidney failure warning: In some cases, this drug can cause your kidneys to stop working. This can occur if you’re on a high dose of this medication and have existing kidney problems. It can also occur if you’re taking other drugs that can harm your kidneys, if you’re not well hydrated, or if you’re over the age of 65 years.
- Effects on the central nervous system warning: If you have kidney disease or use this drug at higher doses than your doctor prescribes, it can build up in your body. High levels of this drug can cause serious side effects that impact your brain. Symptoms can include hallucinations (seeing or hearing things that aren’t real) or delusions (believing things that aren’t true). They can also include agitation, confusion, or seizures. If you have any of these side effects, stop taking this drug. Call 911 right away or go to the nearest emergency room.
Valacyclovir is a prescription drug. It comes in the form of a tablet you take by mouth.
Valacyclovir is available as a brand-name drug called Valtrex. It’s also available as a generic drug. Generic drugs usually cost less than the brand-name version. In some cases, they may not be available in every strength or form as the brand-name drug.
This drug may be used as part of a combination therapy. This means you may need to take it with other medications.
This means you may need to take it with other medications.
Why it’s used
Valacyclovir is used to treat viral infections caused by a group of viruses called herpes simplex viruses. These infections include oral and genital herpes, shingles, and chickenpox.
- Oral herpes causes cold sores. These are small, painful sores that you can get in or around your mouth. Cold sores can be spread by kissing or other physical contact with the infected area of the skin.
- Genital herpes is a sexually transmitted disease. This means it’s spread through sexual contact. Symptoms include small, painful blisters on the genital area. You can spread genital herpes to your sexual partner even when you don’t have any symptoms. This drug is used to treat or prevent flare-ups of genital herpes in people with normal immune systems, or in people with HIV.
- Shinglesis caused by the same virus that causes chickenpox (varicella zoster).
 Symptoms of shingles include small, painful blisters that appear on the skin. Shingles can occur in people who have already had chickenpox. It can also spread to people who have not had chickenpox before through contact with the infected skin.
Symptoms of shingles include small, painful blisters that appear on the skin. Shingles can occur in people who have already had chickenpox. It can also spread to people who have not had chickenpox before through contact with the infected skin. - Chickenpoxcauses an itchy rash of small, red bumps that can look like pimples or insect bites. The rash can spread almost anywhere on the body. Chickenpox can also cause flu-like symptoms, such as fever or tiredness. This drug is used to treat chickenpox in children ages 2 to18 years who have a normal immune system.
How it works
Valacyclovir belongs to a class of drugs called antiviral drugs. A class of drugs is a group of medications that work in a similar way. These drugs are often used to treat similar conditions.
The herpes virus spreads in your body by creating more of its cells. Valacyclovir works by making it harder for the herpes virus to multiply (make more cells) in your body.
This drug does not cure herpes infections. The herpes virus may still live in your body after treatment. This means the infection may occur again at a later time even after the symptoms of the first infection are gone. However, this drug can help prevent such a re-infection at a later time.
Valacyclovir oral tablet doesn’t cause drowsiness, but it can cause other side effects.
More common side effects
The more common side effects of valacyclovir can include:
- headache
- nausea
- vomiting
- dizziness
- pain in your stomach area
If these effects are mild, they may go away within a few days or a couple of weeks. If they’re more severe or don’t go away, talk to your doctor or pharmacist.
Serious side effects
Call your doctor right away if you have serious side effects. Call 911 if your symptoms feel life-threatening or if you think you’re having a medical emergency. Serious side effects and their symptoms can include the following:
- Kidney failure.
 Symptoms can include:
Symptoms can include: - severe drowsiness
- urinating less than usual
- swelling in your legs, ankles, or feet
- Unusual mood or behavior. Symptoms can include:
- aggressive behavior
- unsteady or shaky movements
- confusion
- hallucinations
- seizures
- coma
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs affect each person differently, we cannot guarantee that this information includes all possible side effects. This information is not a substitute for medical advice. Always discuss possible side effects with a healthcare provider who knows your medical history.
Reducing your risk of spreading herpesUsing this medication daily may help lower the risk of spreading this disease to your sexual partner. However, you should not have sexual contact with your partner when you have any symptoms of an outbreak of genital herpes.
Even if you use safer sex practices such as using a condom, you can still spread genital herpes. Talk with your doctor for more information about how to have safer sex.
An interaction is when a substance changes the way a drug works. This can be harmful or prevent the drug from working well. To help prevent interactions, your doctor should manage all of your medications carefully. Be sure to tell your doctor about all medications, vitamins, or herbs you’re taking.
To find out how valacyclovir oral tablet might interact with something else you’re taking, talk to your doctor or pharmacist.
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs interact differently in each person, we cannot guarantee that this information includes all possible interactions. This information is not a substitute for medical advice. Always speak with your healthcare provider about possible interactions with all prescription drugs, vitamins, herbs and supplements, and over-the-counter drugs that you’re taking.
This drug comes with several warnings.
Allergy warning
This drug can cause a severe allergic reaction. Symptoms can include:
- trouble breathing
- swelling of your throat or tongue
If you develop these symptoms, call 911 or go to the nearest emergency room.
Don’t take this drug again if you’ve ever had an allergic reaction to it. Taking it again could be fatal (cause death).
Warnings for people with certain health conditions
For people with kidney problems: Your kidneys clear this drug from your body. If you have kidney problems or a history of kidney disease, you may not be able to clear it from your body. This may increase the levels of the drug in your body and cause more side effects. This drug can also make your kidney function worse. To help prevent these problems, your doctor may prescribe a lower dosage of this drug for you.
For people with advanced HIV or a history of transplant: If you have advanced HIV or a history of bone marrow or kidney transplant, you may be at a higher risk of certain blood disorders. These conditions are called thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). They can result in severely low red blood cells and platelets in your body. TTP or HUS can cause death.
These conditions are called thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). They can result in severely low red blood cells and platelets in your body. TTP or HUS can cause death.
Warnings for other groups
For pregnant women: This drug is a category B pregnancy drug. That means two things:
- Research in animals has not shown a risk to the fetus when the mother takes the drug.
- There aren’t enough studies done in humans to show if the drug poses a risk to the fetus.
Talk to your doctor if you’re pregnant or planning to become pregnant. Animal studies do not always predict the way humans would respond. Therefore, this drug should only be used in pregnancy if clearly needed.
Call your doctor right away if you become pregnant while taking this drug.
For women who are breastfeeding: This drug may pass into breast milk and may cause side effects in a child who is breastfed. Talk to your doctor if you breastfeed your child.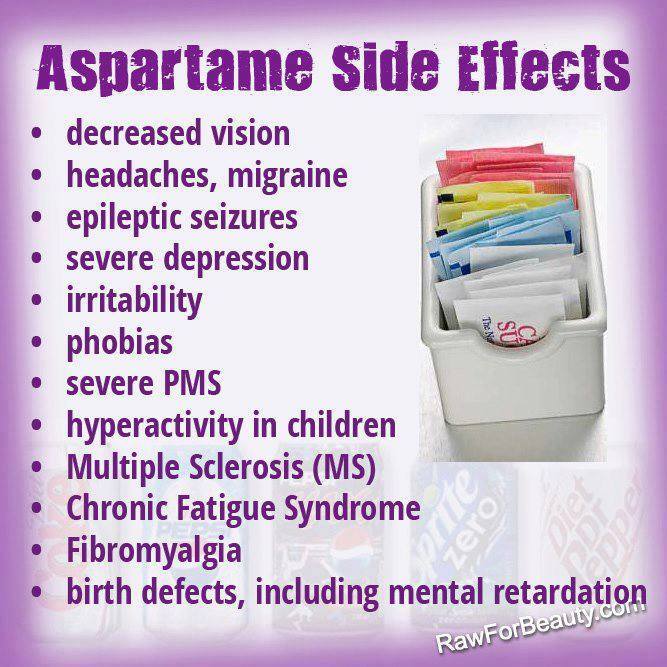 You may need to decide whether to stop breastfeeding or stop taking this medication.
You may need to decide whether to stop breastfeeding or stop taking this medication.
For seniors: The kidneys of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
For children: This drug has not been studied for use in treatment or prevention of herpes simplex virus (HSV) infection in newborn babies. The following are other age limitations for use of this drug:
- Oral herpes (cold sores): This drug has been studied and approved for treatment of cold sores in children ages 12 years and older.
- Genital herpes: This drug has not been studied or approved for treatment of genital herpes in children younger than 18 years.
- Shingles: This drug has not been studied or approved for treatment of shingles in children younger than 18 years.
- Chickenpox: This drug has been studied and approved for treatment of chickenpox in children 2 to 18 years of age. This drug has not been studied or approved for treatment in children younger than 2 years of age.
All possible dosages and drug forms may not be included here. Your dosage, drug form, and how often you take the drug will depend on:
- your age
- the condition being treated
- the severity of your condition
- other medical conditions you have
- how you react to the first dose
Drug forms and strengths
Generic: Valacyclovir
- Form: oral tablet
- Strengths: 500 mg, 1 g
Brand: Valtrex
- Form: oral tablet
- Strengths: 500 mg, 1 g
Dosage for oral herpes
Adult dosage (ages 18–64 years)
- Typical dosage: 2 g, twice per day for 1 day, taken 12 hours apart.
- Note: Treatment should be started at the first sign of cold sore symptoms.
Child dosage (ages 12–17 years)
- Typical dosage: 2 g, twice per day for 1 day, taken 12 hours apart.
- Note: This drug should be started at the first sign of cold sore symptoms.
Child dosage (ages 0–11 years)
- This drug has not been studied or approved for treatment of oral herpes in children younger than 12 years.
Senior dosage (ages 65 years and older)
The kidneys of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
Your doctor may start you on a lowered dosage or a different treatment schedule. This can help keep levels of this drug from building up too much in your body.
Dosage for genital herpes
Adult dosage (ages 18–64 years)
- First episode: 1 g, taken twice per day for 10 days.
 This drug works best if it’s started within 48 hours of when the first symptom appears.
This drug works best if it’s started within 48 hours of when the first symptom appears. - Repeating episodes: 500 mg, taken twice per day for 3 days. Treatment should be started when the first symptom appears.
- For preventing flare-ups in people with a normal immune system: 500 mg to 1 g, taken once per day.
- For preventing flare-ups in people with HIV: 500 mg, taken twice per day.
- For reducing the risk of transmission to a sexual partner: 500 mg, taken once per day.
Child dosage (ages 0–17 years)
This drug has not been studied for the treatment of genital herpes in children younger than 18 years.
Senior dosage (ages 65 years and older)
The kidneys of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
This raises your risk of side effects.
Your doctor may start you on a lowered dosage or a different treatment schedule. This can help keep levels of this drug from building up too much in your body.
Dosage for shingles
Adult dosage (ages 18–64 years)
- Typical dosage: 1 g, taken three times per day for seven days.
- Note: Treatment should be started when the first symptom appears. This drug works best if it’s started within 48 hours of the first sign of a rash on the skin.
Child dosage (ages 0–17 years)
This drug has not been studied for the treatment of shingles in children younger than 18 years.
Senior dosage (ages 65 years and older)
The kidneys of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
Your doctor may start you on a lowered dosage or a different treatment schedule. This can help keep levels of this drug from building up too much in your body.
Dosage for chickenpox
Adult dosage (ages 18–64 years)
- Typical dosage: 1 g, taken 3 times per day for seven days.
- Note: Treatment should be started when the first symptom appears. This drug works best if it’s started within 48 hours of the first sign of a rash on the skin.
Child dosage (ages 2–18 years)
- Typical dosage: 20 mg per kilogram of the child’s body weight, taken 3 times per day for 5 days.
- Maximum dosage: 1 g, taken 3 times per day.
- Note: Treatment should be started at the earliest sign or symptom.
Child dosage (ages 0–1 year)
This drug has not been studied or approved for treatment of chickenpox in children younger than two years.
Senior dosage (ages 65 years and older)
The kidneys of older adults may not work as well as they used to. This can cause your body to process drugs more slowly. As a result, a higher amount of a drug stays in your body for a longer time. This raises your risk of side effects.
Your doctor may start you on a lowered dosage or a different treatment schedule. This can help keep levels of this drug from building up too much in your body.
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs affect each person differently, we cannot guarantee that this list includes all possible dosages. This information is not a substitute for medical advice. Always speak with your doctor or pharmacist about dosages that are right for you.
Valacyclovir oral tablet is used for short-term treatment of oral herpes, genital herpes, shingles, or chickenpox. It’s used for long-term treatment to prevent genital herpes, and to treat genital herpes that recurs (comes back).
This drug comes with serious risks if you don’t take it as prescribed.
If you stop taking the drug suddenly or don’t take it at all: The symptoms of your viral infection may not get better, or may get worse.
If you miss doses or don’t take the drug on schedule: Your medication may not work as well or may stop working completely. If you’re taking this drug to prevent flare-ups of the infection, a certain amount needs to be in your body at all times. You should not stop taking this drug unless your doctor tells you to stop.
If you take too much: You could have dangerous levels of the drug in your body. Symptoms of an overdose of this drug can include more severe side effects, such as:
- headache
- nausea
- tiredness
- dizziness
- diarrhea
- constipation
- weakness or lack of energy
If you think you’ve taken too much of this drug, call your doctor or seek guidance from the American Association of Poison Control Centers at 1-800-222-1222 or through their online tool.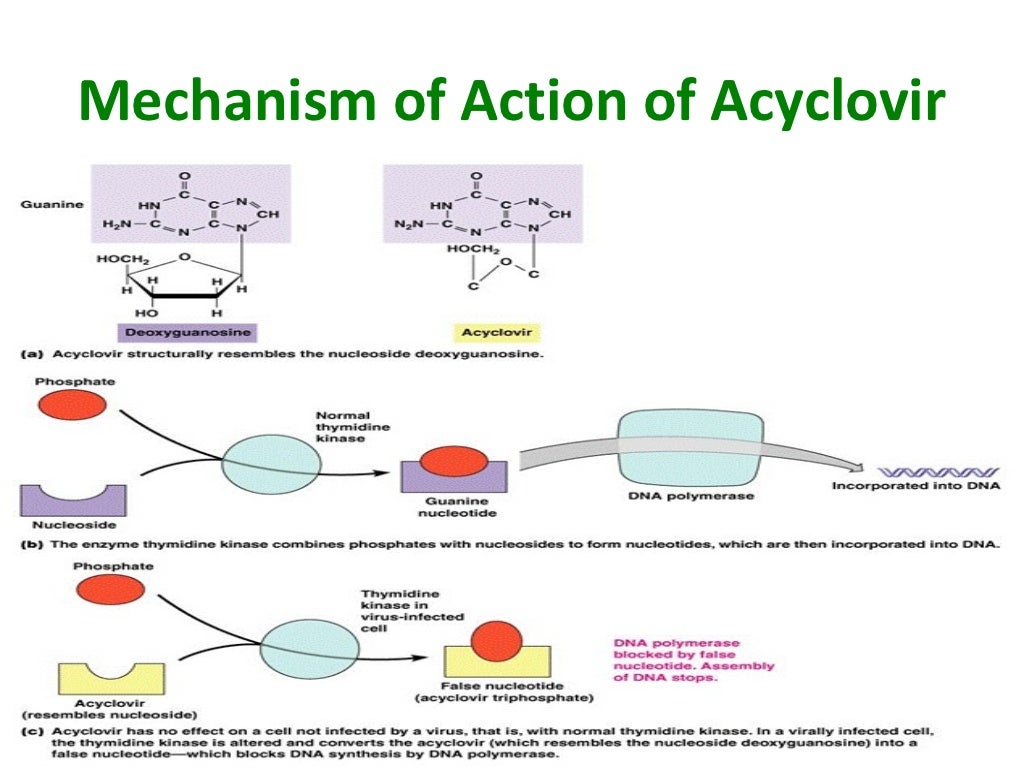 But if your symptoms are severe, call 911 or go to the nearest emergency room right away.
But if your symptoms are severe, call 911 or go to the nearest emergency room right away.
What to do if you miss a dose: Take your dose as soon as you remember. But if you remember just a few hours before your next scheduled dose, take only one dose. Never try to catch up by taking two doses at once. This could result in dangerous side effects.
How to tell if the drug is working: Your symptoms from the viral infection should improve.
Keep these considerations in mind if your doctor prescribes valacyclovir for you.
General
- You can take this drug with or without food. Taking it with food may help reduce any upset stomach.
- Take this drug at the time(s) recommended by your doctor.
Storage
- Store valacyclovir at room temperature between 59°F and 77°F (15°C and 25°C).
- Keep this drug away from light.
- Don’t store this medication in moist or damp areas, such as bathrooms.

Refills
A prescription for this medication is refillable. You should not need a new prescription for this medication to be refilled. Your doctor will write the number of refills authorized on your prescription.
Travel
When traveling with your medication:
- Always carry your medication with you. When flying, never put it into a checked bag. Keep it in your carry-on bag.
- Don’t worry about airport X-ray machines. They can’t harm your medication.
- You may need to show airport staff the pharmacy label for your medication. Always carry the original prescription-labeled container with you.
- Don’t put this medication in your car’s glove compartment or leave it in the car. Be sure to avoid doing this when the weather is very hot or very cold.
Availability
Not every pharmacy stocks this drug. When filling your prescription, be sure to call ahead to make sure your pharmacy carries it.
Hidden costs
You may need to have blood tests during your treatment with this drug. The cost of these tests will depend on your insurance coverage.
The cost of these tests will depend on your insurance coverage.
Prior authorization
Many insurance companies require a prior authorization for this drug. This means your doctor will need to get approval from your insurance company before your insurance company will pay for the prescription.
There are other drugs available to treat your condition. Some may be better suited for you than others. Talk to your doctor about other drug options that may work for you.
Disclaimer: Healthline has made every effort to make certain that all information is factually correct, comprehensive, and up-to-date. However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or other healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
Vayrova. Oral tablets Directory of drugs. Tomsk
//= $single_post["name_eng"] ?> //= $single_post["name_eng"] ?>
Packaging
Oral tabletsPharmacological action
Valaciclovir is a prodrug, rapidly and almost completely converted in the body to aciclovir, which acquires specific activity after phosphorylation. Acyclovir is a structural analogue of purine nucleosides (normal components of DNA), interacts with viral DNA polymerase and blocks the reproduction of viruses. Selective antiherpetic activity is due to affinity for thymidine kinase Herpes simplex, Varicella zoster and Epstein-Barr virus. Under the action of virus thymidine kinase, it is transformed into acyclovir monophosphate, with the participation of human cell guanylate kinase, into acyclovir diphosphate and then into the active form of acyclovir triphosphate. Triphosphate blocks viral DNA replication by competitive inhibition of viral DNA polymerase and inhibition of DNA chain elongation. Acyclovir is active in vitro against Herpes simplex type 1 and type 2 viruses, Varicella zoster (less active than against Herpes simplex due to more efficient phosphorylation by the corresponding thymidine kinase), Epstein-Barr virus, CMV and human herpes virus type 6. nine0003 Indications for use In adults: - herpes of the lips, genital herpes (primary and recurrent infection; long-term suppressive therapy of recurrent genital herpes in people with immunodeficiency, including HIV infection; reduced risk of transmission of infection to a sexual partner), herpes zoster. In adults and children over 12 years of age: - prevention of cytomegalovirus infection in organ transplantation.
Triphosphate blocks viral DNA replication by competitive inhibition of viral DNA polymerase and inhibition of DNA chain elongation. Acyclovir is active in vitro against Herpes simplex type 1 and type 2 viruses, Varicella zoster (less active than against Herpes simplex due to more efficient phosphorylation by the corresponding thymidine kinase), Epstein-Barr virus, CMV and human herpes virus type 6. nine0003 Indications for use In adults: - herpes of the lips, genital herpes (primary and recurrent infection; long-term suppressive therapy of recurrent genital herpes in people with immunodeficiency, including HIV infection; reduced risk of transmission of infection to a sexual partner), herpes zoster. In adults and children over 12 years of age: - prevention of cytomegalovirus infection in organ transplantation. Presentation
film-coated tablets 500 mg; blister 10, cardboard pack 3; film-coated tablets 500 mg; blister 10, cardboard pack 4; film-coated tablets 500 mg; blister 10, cardboard pack 1; film-coated tablets 500 mg; blister 10, cardboard pack 2; nine0003 Pharmacodynamics Resistance of strains of Herpes simplex and Varicella zoster develops due to phenotypic deficiency of viral thymidine kinase, or due to hidden changes in thymidine kinase or DNA polymerase; resistance occurs in exceptional cases in patients with a normal immunological status and much more often against the background of severe immunodeficiency (with HIV infection, in patients receiving chemotherapy for malignant neoplasms, etc. ).
). Pharmacokinetics
After oral administration, valaciclovir is rapidly absorbed from the gastrointestinal tract and, as a result of first-pass metabolism through the intestines and / or liver, due to enzymatic hydrolysis, quickly and almost completely (99%) turns into acyclovir and L-valine. The pharmacokinetics of valacyclovir and acyclovir after oral administration were studied in 14 studies in healthy adult volunteers (n=283). When taking valacyclovir hydrochloride at a dose of 1000 mg, the absolute bioavailability of acyclovir is (54.5 ± 9,1)% and does not depend on food intake. Pharmacokinetic parameters after taking various doses of valaciclovir hydrochloride are not proportional to the dose. So, after a single dose of valaciclovir hydrochloride in doses of 100, 250, 500, 750 and 1000 mg Cmax of acyclovir reaches 0.83; 2.15; 3.28; 4.17 and 5.65 mcg / ml, AUC - 2.28; 5.76; 11.59; 14.11 and 19.52 h µg/ml, respectively. After repeated administration of valacyclovir hydrochloride at doses of 250, 500 and 1000 mg 4 times a day for 11 days, Cmax - 2. 11; 3.69 and 4.96 μg / ml and AUC - 5.66; 9.88 and 15.70 h µg/ml, respectively. Tmax is 1.6–2.1 hours. Plasma concentrations of unchanged valaciclovir are low, Cmax is below 0.5 μg / ml at all doses studied, after 3 hours valaciclovir is no longer detected in plasma; the binding of valaciclovir to plasma proteins is 13.5-17.9%. Acyclovir is biotransformed by alcohol and aldehyde dehydrogenase and, to a lesser extent, aldehyde oxidase into inactive metabolites. The metabolism of valaciclovir/acyclovir is not associated with cytochrome P450 enzymes. T1 / 2 of valaciclovir is less than 30 minutes, T1 / 2 of acyclovir after taking valaciclovir hydrochloride is 2.5-3.3 hours (in healthy volunteers with normal renal function), in elderly patients (65 years-83 years) - 3.3 -3.7 h, increased in patients with end-stage renal disease. Valaciclovir is excreted in the urine (45.6%) and in the faeces (47.12%) within 96 hours Renal clearance - about 255 ml / min. Of the total amount of valacyclovir excreted by the kidneys, more than 80-89% is eliminated as acyclovir.
11; 3.69 and 4.96 μg / ml and AUC - 5.66; 9.88 and 15.70 h µg/ml, respectively. Tmax is 1.6–2.1 hours. Plasma concentrations of unchanged valaciclovir are low, Cmax is below 0.5 μg / ml at all doses studied, after 3 hours valaciclovir is no longer detected in plasma; the binding of valaciclovir to plasma proteins is 13.5-17.9%. Acyclovir is biotransformed by alcohol and aldehyde dehydrogenase and, to a lesser extent, aldehyde oxidase into inactive metabolites. The metabolism of valaciclovir/acyclovir is not associated with cytochrome P450 enzymes. T1 / 2 of valaciclovir is less than 30 minutes, T1 / 2 of acyclovir after taking valaciclovir hydrochloride is 2.5-3.3 hours (in healthy volunteers with normal renal function), in elderly patients (65 years-83 years) - 3.3 -3.7 h, increased in patients with end-stage renal disease. Valaciclovir is excreted in the urine (45.6%) and in the faeces (47.12%) within 96 hours Renal clearance - about 255 ml / min. Of the total amount of valacyclovir excreted by the kidneys, more than 80-89% is eliminated as acyclovir.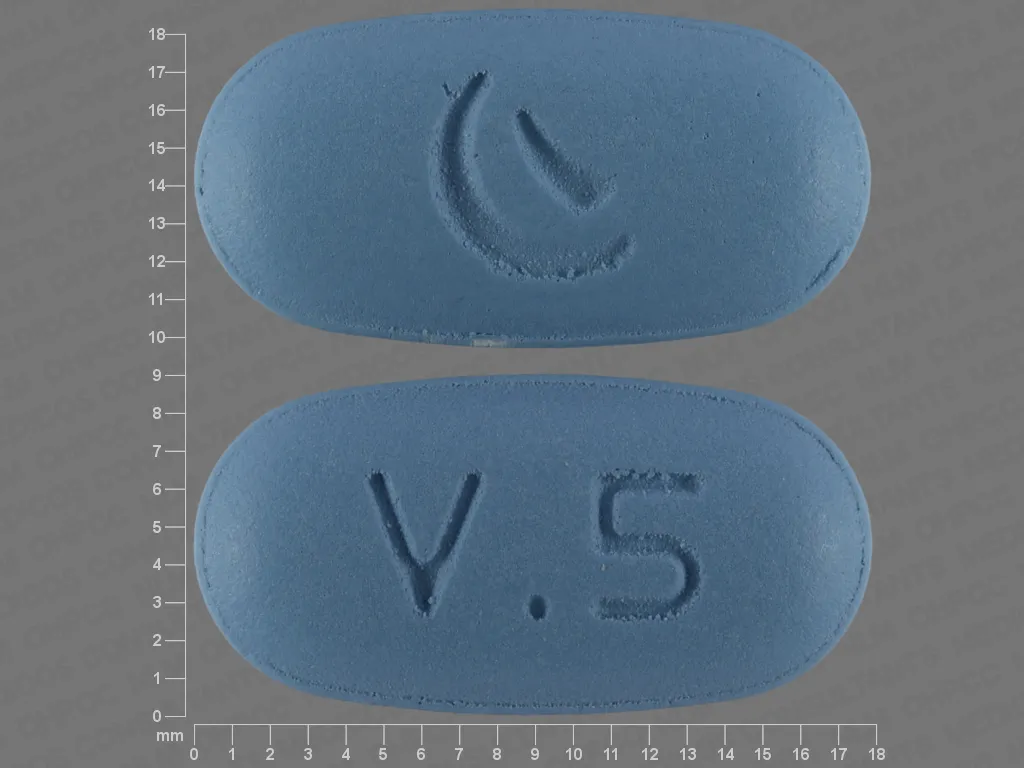 Less than 1% of valaciclovir is excreted unchanged. After repeated use of valacyclovir in patients with normal renal function, acyclovir does not accumulate. Dependence of pharmacokinetic parameters on some factors Liver diseases. In patients with moderate (biopsy-proven cirrhosis) or severe (biopsy-proven cirrhosis with/without ascites) liver disease, the rate, but not the degree, of valaciclovir to acyclovir conversion is reduced; T1 / 2 of acyclovir does not change. HIV-infected patients. 9patients with HIV (number of CD4 cells Teratogenicity. Valaciclovir did not have a teratogenic effect in rats and rabbits when administered at doses of 400 mg / kg during organogenesis (with plasma concentrations exceeding those in humans by 10 and 7 times, respectively). Fertility. Valaciclovir did not cause fertility disorders in male and female rats given a dose of 200 mg/kg/day orally (the concentration in the blood was 6 times higher than that in humans).
Less than 1% of valaciclovir is excreted unchanged. After repeated use of valacyclovir in patients with normal renal function, acyclovir does not accumulate. Dependence of pharmacokinetic parameters on some factors Liver diseases. In patients with moderate (biopsy-proven cirrhosis) or severe (biopsy-proven cirrhosis with/without ascites) liver disease, the rate, but not the degree, of valaciclovir to acyclovir conversion is reduced; T1 / 2 of acyclovir does not change. HIV-infected patients. 9patients with HIV (number of CD4 cells Teratogenicity. Valaciclovir did not have a teratogenic effect in rats and rabbits when administered at doses of 400 mg / kg during organogenesis (with plasma concentrations exceeding those in humans by 10 and 7 times, respectively). Fertility. Valaciclovir did not cause fertility disorders in male and female rats given a dose of 200 mg/kg/day orally (the concentration in the blood was 6 times higher than that in humans). Use during pregnancy
Pregnancy. Perhaps if the expected effect of therapy outweighs the potential risk to the fetus (adequate and well-controlled safety studies in pregnant women have not been conducted). Data on the outcome of pregnancy in women who took systemic acyclovir in the first trimester of pregnancy (acyclovir is an active metabolite of valaciclovir) did not show an increase in the number of congenital malformations in children compared with the general population. Since a small number of women were included in the observation, reliable and definite conclusions about the safety of valacyclovir during pregnancy cannot be made. Category of action on the fetus according to the FDA - B. Lactation. Aciclovir, the main metabolite of valaciclovir, is excreted in breast milk. After the appointment of valaciclovir orally at a dose of 500 mg, Cmax of acyclovir in breast milk was 0.5-2.3 times (on average 1.4 times) higher than the corresponding concentrations in the mother's blood. The ratio of the AUC of acyclovir found in breast milk to the AUC of acyclovir in maternal plasma was 1.
Perhaps if the expected effect of therapy outweighs the potential risk to the fetus (adequate and well-controlled safety studies in pregnant women have not been conducted). Data on the outcome of pregnancy in women who took systemic acyclovir in the first trimester of pregnancy (acyclovir is an active metabolite of valaciclovir) did not show an increase in the number of congenital malformations in children compared with the general population. Since a small number of women were included in the observation, reliable and definite conclusions about the safety of valacyclovir during pregnancy cannot be made. Category of action on the fetus according to the FDA - B. Lactation. Aciclovir, the main metabolite of valaciclovir, is excreted in breast milk. After the appointment of valaciclovir orally at a dose of 500 mg, Cmax of acyclovir in breast milk was 0.5-2.3 times (on average 1.4 times) higher than the corresponding concentrations in the mother's blood. The ratio of the AUC of acyclovir found in breast milk to the AUC of acyclovir in maternal plasma was 1. 4–2.6 (average 2.2). The average concentration of acyclovir in breast milk is 2.24 μg / ml. When a nursing mother is given valaciclovir 500 mg twice daily, the child will be exposed to the same effects of aciclovir as when taken orally at a dose of about 0.61 mg/kg/day. Valaciclovir unchanged is not detected in the blood and breast milk of the mother or the urine of the child. Valaciclovir should be administered to nursing women with caution, only if necessary. nine0003 Contraindications for use Hypersensitivity (including to acyclovir), bone marrow transplantation, kidney transplantation.
4–2.6 (average 2.2). The average concentration of acyclovir in breast milk is 2.24 μg / ml. When a nursing mother is given valaciclovir 500 mg twice daily, the child will be exposed to the same effects of aciclovir as when taken orally at a dose of about 0.61 mg/kg/day. Valaciclovir unchanged is not detected in the blood and breast milk of the mother or the urine of the child. Valaciclovir should be administered to nursing women with caution, only if necessary. nine0003 Contraindications for use Hypersensitivity (including to acyclovir), bone marrow transplantation, kidney transplantation. Side effects
From the digestive system: nausea, abdominal pain, vomiting, diarrhea, increased activity of ALT, AST, alkaline phosphatase, hepatitis. From the nervous system: headache, dizziness, depression, aggressive behavior, agitation, ataxia, coma, confusion or depression of consciousness, dysarthria, encephalopathy, mania, psychosis, incl. auditory and visual hallucinations, convulsions, tremors. From the senses: blurred vision. From the urinary system: pain in the projection of the kidneys, acute renal failure. On the part of the hematopoietic organs: neutropenia, thrombocytopenia, aplastic anemia, leukoclastic vasculitis, thrombotic thrombocytopenic purpura. On the part of the skin: erythema multiforme, rash, photosensitivity, alopecia. Allergic reactions: angioedema, shortness of breath, itching, rash, urticaria, anaphylactic reactions. Laboratory indicators: decrease in Hb, hypercreatininemia. Others: dysmenorrhea, arthralgia, nasopharyngitis, respiratory infections, facial edema, increased blood pressure, tachycardia, fatigue; additionally in children - fever, dehydration, rhinorrhea. nine0003 Method of administration and dosage Inside, regardless of food intake. Adults, incl. with chronic renal failure and CC less than 50 ml / min / 1.73 sq.m: With herpes of the lips (treatment begins with the appearance of tingling, itching or burning) - 2 g every 12 hours, with CC 30-49 - 1 g every 12 hours, with CC 10-29 - 500 mg every 12 hours, with CC less than 10 - 500 mg once.
From the senses: blurred vision. From the urinary system: pain in the projection of the kidneys, acute renal failure. On the part of the hematopoietic organs: neutropenia, thrombocytopenia, aplastic anemia, leukoclastic vasculitis, thrombotic thrombocytopenic purpura. On the part of the skin: erythema multiforme, rash, photosensitivity, alopecia. Allergic reactions: angioedema, shortness of breath, itching, rash, urticaria, anaphylactic reactions. Laboratory indicators: decrease in Hb, hypercreatininemia. Others: dysmenorrhea, arthralgia, nasopharyngitis, respiratory infections, facial edema, increased blood pressure, tachycardia, fatigue; additionally in children - fever, dehydration, rhinorrhea. nine0003 Method of administration and dosage Inside, regardless of food intake. Adults, incl. with chronic renal failure and CC less than 50 ml / min / 1.73 sq.m: With herpes of the lips (treatment begins with the appearance of tingling, itching or burning) - 2 g every 12 hours, with CC 30-49 - 1 g every 12 hours, with CC 10-29 - 500 mg every 12 hours, with CC less than 10 - 500 mg once. The course of treatment - 1 day; With primary genital herpes (including with CC 30-49 ml / min / 1.73 sq.m) - 1 g 2 times a day, with CC 10-29 - 1 g every 24 hours, with CC less than 10 - 500 mg every 24 hours. The course of treatment is 10 days; With recurrent genital herpes (including with CC 30-49ml / min / 1.73 sq.m) - 500 mg 2 times a day, with CC less than 30 - 500 mg every 24 hours. The course of treatment is 3 days. Treatment begins at the first appearance of signs or symptoms of relapse; With long-term suppressive therapy of recurrent genital herpes in immunocompromised individuals, incl. HIV infection - 1 g per day (including with CC 30-49), with CC less than 30 - 500 mg every 24 hours, with a number of relapses of less than 9 per year, use is possible (including with CC 30-49) - 500 mg per day, with CC less than 30 - 500 mg every 48 hours, in HIV-infected people with more than 100 CD4 + cells / μl (including with CC 30-49) - 500 mg 2 times a day, with CC less than 30 - 500 mg every 24 hours; To reduce the risk of transmission of infection to a sexual partner with a number of relapses of less than 9 per year, the sexual partner is prescribed 500 mg 1 time per day.
The course of treatment - 1 day; With primary genital herpes (including with CC 30-49 ml / min / 1.73 sq.m) - 1 g 2 times a day, with CC 10-29 - 1 g every 24 hours, with CC less than 10 - 500 mg every 24 hours. The course of treatment is 10 days; With recurrent genital herpes (including with CC 30-49ml / min / 1.73 sq.m) - 500 mg 2 times a day, with CC less than 30 - 500 mg every 24 hours. The course of treatment is 3 days. Treatment begins at the first appearance of signs or symptoms of relapse; With long-term suppressive therapy of recurrent genital herpes in immunocompromised individuals, incl. HIV infection - 1 g per day (including with CC 30-49), with CC less than 30 - 500 mg every 24 hours, with a number of relapses of less than 9 per year, use is possible (including with CC 30-49) - 500 mg per day, with CC less than 30 - 500 mg every 48 hours, in HIV-infected people with more than 100 CD4 + cells / μl (including with CC 30-49) - 500 mg 2 times a day, with CC less than 30 - 500 mg every 24 hours; To reduce the risk of transmission of infection to a sexual partner with a number of relapses of less than 9 per year, the sexual partner is prescribed 500 mg 1 time per day. With herpes zoster - 1 g every 8 hours for 7 days (treatment begins within 48 hours after the onset of the rash), with CC 30-49 - 1 g every 12 hours, with CC 10-29 - 1 g each every 24 hours With CC less than 10 - 500 mg every 24 hours. Adults and children over 12 years old, incl. with chronic renal failure and CC less than 75 ml / min / 1.73 sq.m: prevention of CMV infection during organ transplantation - 2 g 4 times a day; with CC 50-75 - 1.5 g 4 times a day; with CC 25-50 - 1.5 g 3 times a day; with CC 10-25 - 1.5 g 2 times a day; with CC less than 10 and patients on hemodialysis - 1.5 g 1 time per day. Course of treatment - 90 days. The drug is started as soon as possible after the transplant. For patients on hemodialysis, the drug is administered after the hemodialysis procedure. T1 / 2 in such patients - 4 hours; during the 4-hour procedure, 30% of the drug is removed. With peritoneal dialysis, the drug is removed to a lesser extent, other pharmacokinetic parameters remain the same.
With herpes zoster - 1 g every 8 hours for 7 days (treatment begins within 48 hours after the onset of the rash), with CC 30-49 - 1 g every 12 hours, with CC 10-29 - 1 g each every 24 hours With CC less than 10 - 500 mg every 24 hours. Adults and children over 12 years old, incl. with chronic renal failure and CC less than 75 ml / min / 1.73 sq.m: prevention of CMV infection during organ transplantation - 2 g 4 times a day; with CC 50-75 - 1.5 g 4 times a day; with CC 25-50 - 1.5 g 3 times a day; with CC 10-25 - 1.5 g 2 times a day; with CC less than 10 and patients on hemodialysis - 1.5 g 1 time per day. Course of treatment - 90 days. The drug is started as soon as possible after the transplant. For patients on hemodialysis, the drug is administered after the hemodialysis procedure. T1 / 2 in such patients - 4 hours; during the 4-hour procedure, 30% of the drug is removed. With peritoneal dialysis, the drug is removed to a lesser extent, other pharmacokinetic parameters remain the same.
Overdose
Symptoms: sedimentation of acyclovir in the renal tubules. Treatment: hemodialysis (for acute renal failure and anuria). nine0003 Interaction with other drugs No clinically significant interactions have been established in patients with normal renal function. Cimetidine and probenecid (singly or together) after taking valacyclovir in a single dose of 1000 mg increase Cmax and AUC and reduce the renal clearance of acyclovir. The pharmacokinetics of valacyclovir does not change when taken simultaneously with digoxin, aluminum / magnesium-containing antacids, thiazide diuretics. Nephrotoxic drugs increase the risk of developing renal failure and disorders of the central nervous system. nine0003 Precautions Use with great caution in any condition accompanied by immunodeficiency, especially in HIV-infected patients. During the period of clinical trials, when taking valaciclovir in high doses (8 g / day) for a long time, thrombocytopenic purpura and / or hemolytic uremic syndrome were recorded, in rare cases with a fatal outcome, in patients with clinically pronounced forms of HIV infection, after transplantation bone marrow or kidney. The efficacy and safety of the use of valaciclovir in immunocompromised patients (with the exception of suppressive therapy of genital herpes in HIV-infected patients), for suppressive therapy of recurrent genital herpes in patients with clinically severe forms of HIV infection (CD4 cell count) have not been established. It is used with caution in case of impaired renal function (T1 / 2 of acyclovir lengthens), correction of the dosing regimen is necessary depending on the degree of impairment of their function (see "Method of application and dose"). In patients with kidney disease receiving high doses of valaciclovir, there have been cases of acute renal failure and disorders of the central nervous system. Elderly patients during treatment are advised to increase the amount of fluid consumed. During the treatment of genital herpes, sexual intercourse should be avoided (the drug does not protect against transmission of infection). nine0003 Special instructions for taking Elderly patients, persons with dehydration during the treatment period, it is necessary to increase the amount of fluid consumed (risk of developing acute renal failure).
The efficacy and safety of the use of valaciclovir in immunocompromised patients (with the exception of suppressive therapy of genital herpes in HIV-infected patients), for suppressive therapy of recurrent genital herpes in patients with clinically severe forms of HIV infection (CD4 cell count) have not been established. It is used with caution in case of impaired renal function (T1 / 2 of acyclovir lengthens), correction of the dosing regimen is necessary depending on the degree of impairment of their function (see "Method of application and dose"). In patients with kidney disease receiving high doses of valaciclovir, there have been cases of acute renal failure and disorders of the central nervous system. Elderly patients during treatment are advised to increase the amount of fluid consumed. During the treatment of genital herpes, sexual intercourse should be avoided (the drug does not protect against transmission of infection). nine0003 Special instructions for taking Elderly patients, persons with dehydration during the treatment period, it is necessary to increase the amount of fluid consumed (risk of developing acute renal failure).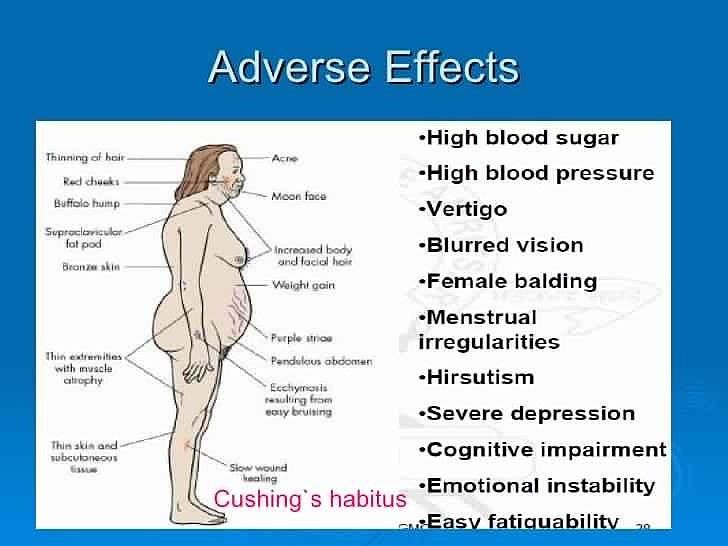 In the treatment of genital herpes, sexual intercourse should be avoided, because. the drug does not protect against transmission of infection. Taking the drug in high doses for a long time in conditions accompanied by severe immunodeficiency (bone marrow transplantation, clinically pronounced forms of HIV infection, kidney transplantation) led to the development of thrombocytopenic purpura and hemolytic uremic syndrome, up to death. If side effects occur from the side of the central nervous system (including agitation, hallucinations, confusion, delirium, convulsions and encephalopathy), the drug is canceled. nine0003 Storage conditions List B.: In a dry, dark place, at a temperature not exceeding 25 °C.
In the treatment of genital herpes, sexual intercourse should be avoided, because. the drug does not protect against transmission of infection. Taking the drug in high doses for a long time in conditions accompanied by severe immunodeficiency (bone marrow transplantation, clinically pronounced forms of HIV infection, kidney transplantation) led to the development of thrombocytopenic purpura and hemolytic uremic syndrome, up to death. If side effects occur from the side of the central nervous system (including agitation, hallucinations, confusion, delirium, convulsions and encephalopathy), the drug is canceled. nine0003 Storage conditions List B.: In a dry, dark place, at a temperature not exceeding 25 °C. Shelf life
36 months.instructions for use, classification, articles » Directory of drugs
Description: Valaciclovir tablets: 500mg: Blue tablets, rectangular with rounded corners, biconvex surface, there is a whisk, the inscription "C 324 500" on one side and smooth on the other sides.
Composition: Each tablet contains:
active substances: valaciclovir (as valaciclovir hydrochloride) 500 mg;
excipients: microcrystalline cellulose, crospovidone, hypromellose, magnesium stearate, Opadry blue 13B50647;
Composition Opadray blue 13B50647; hypromellose, titanium dioxide, macrogol/polyethylene glycol 400, indigo carmine aluminum lacquer, polysorbate 80.
Mechanism of action: Valaciclovir is a nucleoside analogue DNA polymerase inhibitor. Valaciclovir hydrochloride is rapidly converted to acyclovir, which has demonstrated antiviral activity against HSV types 1 (HSV-1) and 2 (HSV-2) and VZV in cell culture and in vivo. nine0035
The inhibitory activity of acyclovir is highly selective due to its proximity to the enzyme thymidine kinase (G) encoded by HSV and VZV. This viral enzyme converts acyclovir to acyclovir monophosphate nucleotide analog. Monophosphate is further converted to diphosphate by cellular guanylate kinase and to triphosphate by a number of cellular enzymes.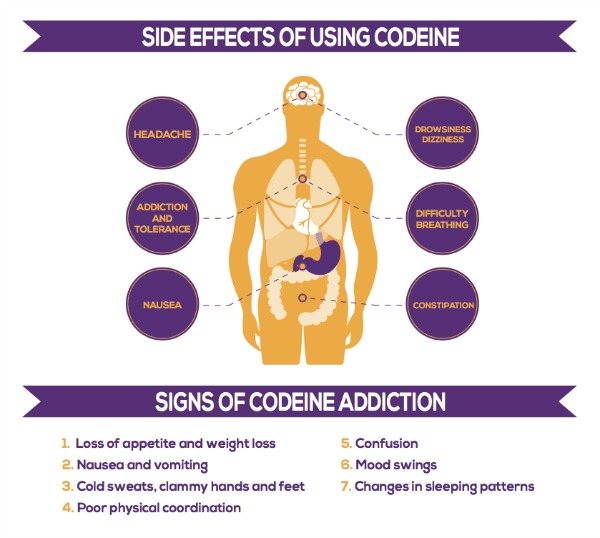 In biochemical assays, acyclovir triphosphate inhibits the replication of herpes viral DNA. This is achieved in 3 ways: 1) competitive inhibition of the viral DNA polymerase, 2) turning on and terminating the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV, compared with VZV, is associated with its more efficient phosphorylation by viral MCs. nine0035
In biochemical assays, acyclovir triphosphate inhibits the replication of herpes viral DNA. This is achieved in 3 ways: 1) competitive inhibition of the viral DNA polymerase, 2) turning on and terminating the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV, compared with VZV, is associated with its more efficient phosphorylation by viral MCs. nine0035
Antiviral activity: A quantitative relationship between herpes virus cell culture susceptibility to antivirals and clinical response to therapy has not been established in humans, and virus susceptibility testing has not been standardized. Susceptibility test results, expressed as the concentration of drug required to inhibit up to 50% of virus growth in cell culture (EC 50 ), vary greatly depending on a number of factors. Using platelet reduction tests, it was determined that EC 50 values against herpes simplex virus isolates range from 0. 09 to 60 µM (0.02 to 13.5 µg/mL), for HSV-1 and from 0.04 to 44 µM (0.01 up to 9.9 µg/ml), for HSV-2 EU 50 values for aciclovir against most laboratory strains and VZV clinical isolates range from 0.53 to 48 µM (0.12 to 10.8 µg/ml ). Aciclovir also shows activity against the vaccine strain Oka VZV with an average EC 50 6 µM (1.35 µg/ml). nine0035
09 to 60 µM (0.02 to 13.5 µg/mL), for HSV-1 and from 0.04 to 44 µM (0.01 up to 9.9 µg/ml), for HSV-2 EU 50 values for aciclovir against most laboratory strains and VZV clinical isolates range from 0.53 to 48 µM (0.12 to 10.8 µg/ml ). Aciclovir also shows activity against the vaccine strain Oka VZV with an average EC 50 6 µM (1.35 µg/ml). nine0035
Resistance: HSV and VZV resistance to aciclovir may result from qualitative and quantitative changes in viral TK and/or DNA polymerase. Clinical VZV isolates with reduced susceptibility to acyclovir have been found in patients with AIDS. In these cases, TK-deficient VZV mutants were recovered.
HSV and VZV resistance to acyclovir occurs through the same mechanisms. Although most acyclovir-resistant mutants have been isolated, immunocompromised patients are TK-deficient mutants. Other mutants associated with the viral TK gene (TK partial and TK altered), DNA polymerases were also isolated. TK-negative mutants can cause serious disease in immunocompromised patients. The possibility of viral resistance to valacyclovir (and therefore to acyclovir) should be considered in patients who show a poor clinical response during therapy. nine0035
The possibility of viral resistance to valacyclovir (and therefore to acyclovir) should be considered in patients who show a poor clinical response during therapy. nine0035
Pharmacokinetics in adults:
Absorption and bioavailability: After oral administration, valaciclovir hydrochloride is rapidly absorbed in the gastrointestinal tract and almost completely converted to acyclovir and L-valine as a result of first-pass metabolism through the intestine and / or hepatic metabolism.
The absolute bioavailability of acyclovir following administration of valaciclovir hydrochloride is 54.5% ± 9.1%, as determined following a dose of 1 gram of valaciclovir hydrochloride and 350 mg of intravenous aciclovir in 12 healthy volunteers. The bioavailability of acyclovir when taking valaciclovir hydrochloride does not change when taken with food (30 minutes after breakfast at 873 kcal, containing 51 g of fat). nine0035
Pharmacokinetic parameters of acyclovir assessing subsequent administration of valaciclovir hydrochloride in healthy adult volunteers are presented in Table 1. There was a smaller dose-proportional increase in the maximum concentration of acyclovir (C m ax ) and the area of the pharmacokinetic curve of acyclovir (AUC) after single and multiple dosing doses (4 times a day) of valaciclovir hydrochloride from 250 mg to 1 gram.
There was a smaller dose-proportional increase in the maximum concentration of acyclovir (C m ax ) and the area of the pharmacokinetic curve of acyclovir (AUC) after single and multiple dosing doses (4 times a day) of valaciclovir hydrochloride from 250 mg to 1 gram.
No accumulation of acyclovir has been observed following administration of valaciclovir at the recommended dosage regimens in adult patients with normal renal function. nine0035
Table 1. Mean (± standard deviation (SD)) plasma pharmacokinetic parameters of aciclovir after administration of valaciclovir hydrochloride in healthy adult volunteers
| Dose | Single dose | Multiple doses* | ||
| AUC (±SD) (h*mcg/ml) | Cmax (±SD) | AUC (±SD) | ||
| 100mg | 0. | 2.28 (±0.40) | ND nine0110 | ND |
| 250mg | 2.15 (±0.50) | 5.76 (±0.60) | 2.11 (±0.33) | 5.66 (±1.09) |
| 500mg | 3.28 (±0.83) | nine0123 3.69 (±0.87) | 9.88 (±2.01) | |
| 750mg | 4.17 (±0.14) | 14.11 (±3.54) | ND | ND |
nine0108 1. 000mg 000mg | 5.65 (±2.37) | 19.52 (±6.04) | 4.96 (±0.64) | 15.70 (±2.27) |
*Taken 4 times a day for 11 days.
ND = not done.
Distribution: The binding of valaciclovir to human plasma proteins ranges from 13.5% to 17.9%. The binding of acyclovir to human plasma proteins varies from 9% to 33%.
Metabolism: Valaciclovir is converted to acyclovir and L-valine by first-pass intestinal and/or hepatic metabolism. Aciclovir is to a small extent converted to inactive metabolites by aldehyde oxidase, alcohol and aldehyde dehydrogenase. Neither acyclovir nor valaciclovir are metabolized by cytochrome P450 enzymes. Plasma concentrations of unreacted valaciclovir are low and transient, usually not quantifiable after 3 hours of administration. Peak plasma concentrations of valaciclovir are typically less than 0. 5 µg/mL at all doses. Following a single dose of 1 gram of valaciclovir hydrochloride, observed mean plasma concentrations of valaciclovir were 0.5, 0.4, and 0.8 µg/ml in patients with hepatic impairment, renal insufficiency, and in healthy volunteers treated with concomitant cimetidine and probenecid, respectively. nine0035
5 µg/mL at all doses. Following a single dose of 1 gram of valaciclovir hydrochloride, observed mean plasma concentrations of valaciclovir were 0.5, 0.4, and 0.8 µg/ml in patients with hepatic impairment, renal insufficiency, and in healthy volunteers treated with concomitant cimetidine and probenecid, respectively. nine0035
Elimination: Following oral administration of a single dose of 1 gram of radioactively labeled valaciclovir to 4 healthy subjects, 46% and 47% of the administered radioactivity was found in urine and feces, respectively, after more than 96 hours. Acyclovir accounted for 89% of the radioactivity excreted in the urine. The renal clearance of aciclovir after a single dose of 1 gram of valaciclovir hydrochloride in 12 healthy volunteers was approximately 255 ± 86 ml/min, which is 42% of the total apparent plasma clearance of aciclovir. nine0035
The plasma half-life of acyclovir is typically 2.5 to 3.3 hours in all studies of valaciclovir hydrochloride in volunteers with normal renal function.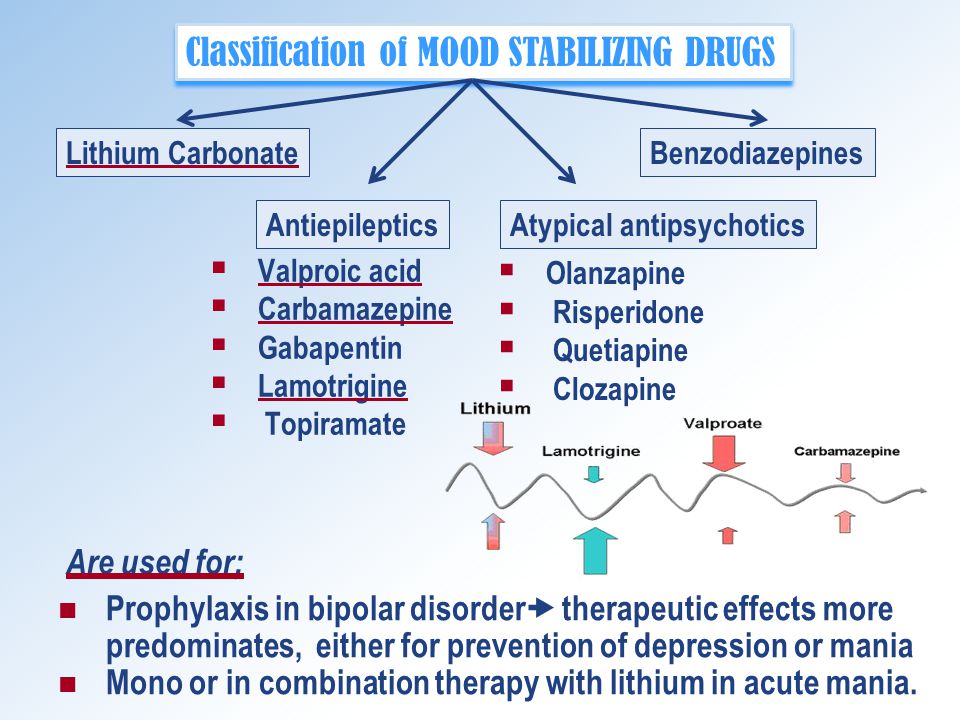
Specific Populations:
Renal Insufficiency: For patients with renal insufficiency, dose reduction is recommended. Following administration of valacyclovir hydrochloride to volunteers with chronic renal failure, the mean elimination half-life of acyclovir is approximately 14 hours. During hemodialysis, the half-life of acyclovir is about 4 hours. Approximately one third of the acyclovir in the body is removed by dialysis during a 4-hour hemodialysis session. The apparent plasma clearance of acyclovir in dialysis patients was 86.3 ± 21.3 ml / min / 1.73 m 2 compared with 679.16 ± 162.76 ml/min/1.73m 2 in healthy volunteers.
Liver injury: Administration of valaciclovir hydrochloride to patients with moderate (biopsy-proven cirrhosis) or severe (with or without ascites and biopsy-confirmed cirrhosis) liver disease has shown that the rate, but not extent, of the conversion of valaciclovir to aciclovir is reduced, and the period The half-life of acyclovir does not change. For patients with cirrhosis of the liver, a change in dosage is not recommended. nine0035
For patients with cirrhosis of the liver, a change in dosage is not recommended. nine0035
HIV infection: In 9 patients with HIV infection and CD4+ 3 cells who received valacyclovir hydrochloride at a dose of 1 g 4 times a day for 30 days, the pharmacokinetics of valaciclovir and acyclovir did not differ from that observed in healthy volunteers.
Geriatrics: Following a single dose of 1 gram of valaciclovir hydrochloride in healthy geriatric volunteers, the elimination half-life of acyclovir was 3.11 ± 0.51 hours compared with 2.91 ± 0.63 hours in healthy young volunteers. The pharmacokinetics of aciclovir after oral administration of single and multiple doses of valaciclovir hydrochloride in geriatric volunteers varied with renal function. Dose reduction may be required in geriatric patients depending on the state of renal function. nine0035
Pediatrics: The pharmacokinetics of aciclovir was evaluated in a total of 98 pediatric patients (range 1 month to
Table 2. ( ± SD ) Pharmacokinetic parameters of acyclovir in plasma after receiving the first dose 20 mg/kg of the periral suspension of the pediatric patients in the pediatric of 1 gram. hydrochloride in adult patients
( ± SD ) Pharmacokinetic parameters of acyclovir in plasma after receiving the first dose 20 mg/kg of the periral suspension of the pediatric patients in the pediatric of 1 gram. hydrochloride in adult patients
| Parameter | Pediatric patients (20 mg/kg oral suspension) | Adult patients 1 gram solid dose of valaciclovir hydrochloride (N=15) | ||
| 1- | 2- | 6- | AUC (µg*h/ml) | 14.4 (±6.26) | 10.1 (±3.35) | 13.1 (±3.43) | 17.2 (±3.10) |
| Cmax (µg/ml) | 4. | 3.75 (±1.14) | nine0123 4.72 (±1.37) | |
Drug Interactions : When valaciclovir hydrochloride is co-administered with antacids, cimetidine and/or probenicide, digoxin or thiazide diuretics in patients with normal renal function, effects are not considered clinically significant (see below). Therefore, when valaciclovir hydrochloride is co-administered with these drugs in patients with normal renal function, dose adjustment is not required. nine0035
Antacids: The pharmacokinetics of acyclovir after a single dose of valaciclovir hydrochloride (1 g) remained unchanged while taking a single dose of antacids (Al + and Mg + ).
Cimetidine: C m ax and AUC of aciclovir after a single dose of valaciclovir hydrochloride (1g) increased by 8% and 32%, respectively, after taking a single dose of cimetidine (800mg).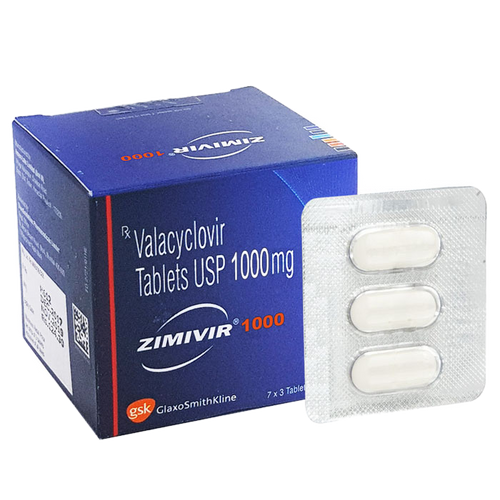
Cimetidine plus Probenecid: C m ax and AUC of acyclovir after a single dose of valacyclovir hydrochloride (1 g) increased by 30% and 78%, respectively, after taking the combination of cimetidine and probenecid, mainly due to a decrease in the renal clearance of acyclovir.
Digoxin: The pharmacokinetics of digoxin was not affected by concomitant administration of valaciclovir hydrochloride 1 g 3 times a day, the pharmacokinetics of aciclovir after a single dose of valaciclovir hydrochloride (1 g) was not affected by concomitant administration of digoxin (2 doses of 0.75 mg). nine0035
Probenecid: C m ax and AUC of acyclovir after a single dose of valaciclovir hydrochloride (1 g) increased by 22%) and 49%), respectively, after taking probenecid (1 gram).
Thiazide diuretics: The pharmacokinetics of acyclovir after a single dose of valaciclovir hydrochloride (1 g) remained unchanged when several doses of thiazide diuretics were taken simultaneously.
Adult patients:
Herpes simplex (lip cold sores): Valaciclovir tablets are for the treatment of herpes simplex (lip cold sores). The effectiveness of valaciclovir hydrochloride, starting from the development of clinical manifestations of herpes simplex (eg, papules, vesicles or ulcers), has not been established.
Genital herpes:
First episode: Valaciclovir tablets are indicated for the treatment of the initial period of genital herpes in immunocompetent adults. The effectiveness of treatment with valaciclovir hydrochloride 72 hours after the onset of symptoms has not been established. nine0035
Recurrent genital herpes: Valaciclovir tablets are indicated for the treatment of relapses of genital herpes in immunocompetent adults. The effectiveness of treatment with valaciclovir hydrochloride more than 24 hours after the onset of symptoms has not been established.
Suppressive therapy for genital herpes: Valaciclovir tablets are indicated for the suppressive treatment of chronic recurrent episodes of genital herpes in immunocompetent patients and in HIV-infected adults. The efficacy and safety of valaciclovir hydrochloride for the suppression of genital herpes at 1 year in immunocompetent patients and at 6 months in HIV-infected patients has not been established. nine0035
The efficacy and safety of valaciclovir hydrochloride for the suppression of genital herpes at 1 year in immunocompetent patients and at 6 months in HIV-infected patients has not been established. nine0035
Reducing the risk of transmission of genital herpes: Valaciclovir tablets are indicated to reduce the transmission of genital herpes in immunocompetent adults. The efficacy of valaciclovir hydrochloride in reducing the transmission of genital herpes at 8 months in discordant couples has not been established. The effectiveness of valaciclovir hydrochloride in reducing the transmission of genital herpes in people with multiple partners and in non-heterosexual couples has not been established.
Herpes zoster : Valaciclovir tablets are indicated for the treatment of herpes zoster (shingles) in immunocompetent adults. The efficacy of valaciclovir hydrochloride when used more than 72 hours after the onset of the rash, and the efficacy and safety of valaciclovir hydrochloride for the treatment of herpes zoster, have not been established.
Pediatric use of :
Herpes simplex (labial herpes): Valaciclovir tablets are indicated for the treatment of herpes simplex (labial herpes) in pediatric patients 12 years of age and older. The effectiveness of valaciclovir hydrochloride after the development of clinical manifestations of herpes simplex (eg, papules, vesicles or ulcers) has not been established.
Varicella : Valaciclovir tablets are indicated for the treatment of varicella in immunocompetent pediatric patients 2 years of age and older. Treatment with valaciclovir tablets should begin within 24 hours of the onset of the rash. nine0035
Restrictions on the use of the drug:
Efficacy and safety of valaciclovir tablets have not been established for:
- Immunocompromised patients, except for the suppression of genital herpes in HIV-infected patients with CD4+ cells >100 cells/mm3.
- Patients under 12 years of age with herpes simplex (cold sores of the lips).

- Patients under 2 years of age and over 18 years of age with chickenpox. nine0505
- Patients under 18 years of age with genital herpes.
- Patients under 18 years of age with herpes zoster.
- Neonates and infants on suppressive therapy for herpes simplex virus.
Valaciclovir tablets may be taken without food. Valaciclovir oral suspension (25 mg/ml and 50 mg/ml) can be prepared from valaciclovir 500 mg tablets for use in pediatric patients for whom a solid dosage form is not suitable. nine0035
Adult dosage:
Herpes simplex (lip cold sores): The recommended dose of valaciclovir hydrochloride for the treatment of herpes simplex is 2 grams twice a day for 1 day at intervals of 12 hours. Therapy should be started as soon as possible when signs of herpes (eg, tingling, itching, or burning) appear.
Genital herpes:
First episode: The recommended dosage of valaciclovir hydrochloride for the initial treatment of genital herpes is 1 gram twice a day for 10 days. Therapy is most effective when taken by mouth within 48 hours of the onset of signs and symptoms of the disease. nine0035
Therapy is most effective when taken by mouth within 48 hours of the onset of signs and symptoms of the disease. nine0035
Intermittent episodes: The recommended dosage of valaciclovir hydrochloride for the treatment of recurrent genital herpes is 500 mg twice daily for 3 days. Treatment must begin at the first sign of the disease.
Suppressive therapy: The recommended dose of valaciclovir hydrochloride for chronic suppressive therapy of recurrent genital herpes is 1 gram once daily in patients with normal immune function. Patients with a history of less than 9relapses per year, the alternative dose is 500 mg once daily.
In HIV-infected patients with CD4+ cells ≥100 cells/mm 3 The recommended dose of valaciclovir hydrochloride for chronic suppressive treatment of recurrent genital herpes is 500 mg twice daily.
Reducing the risk of transmission: The recommended dose of valaciclovir hydrochloride to reduce the transmission of genital herpes in patients with a history of no more than 9 relapses per year is 500 mg once daily for the carrier partner. nine0035
nine0035
Herpes zoster: The recommended dosage of valaciclovir hydrochloride for the treatment of herpes zoster is 1 gram 3 times a day for 7 days. Therapy should be started as soon as possible when signs or symptoms of herpes zoster appear and are most effective when started within 48 hours of the onset of the rash.
Pediatric dosage:
Herpes simplex (cold sores of the lips): The recommended dose of valaciclovir hydrochloride for the treatment of herpes in children 12 years of age and older is 2 grams twice a day for 1 day at 12 hour intervals. Therapy should be initiated as soon as possible when signs of herpes simplex (eg, tingling, itching, or burning) appear.
Varicella: The recommended dosage of valaciclovir hydrochloride for the treatment of varicella in immunocompetent pediatric patients aged 2 to 18 years is 20 mg/kg 3 times a day for 5 days. The total dose should not exceed 1 gram taken 3 times a day. Therapy should be started as soon as possible when signs or symptoms of the disease appear. nine0035
nine0035
Patients with renal insufficiency:
Dosing recommendations for adult patients with reduced renal function are presented in Table 3 (recommendations are not suitable for the use of valaciclovir hydrochloride in pediatric patients with creatinine clearance 2).
Table 3 Dosing recommendations for valaciclovir hydrochloride in adult patients with renal insufficiency
| Normal dosing regimen (creatinine clearance >50ml/min) | Creatinine clearance (ml/min) | |||
| 30-49 | 10-29 | |||
| Herpes simplex (Herpes of the lips). Treatment should not be more than 1 day. nine0035 | Two doses of 2 grams 12 hours apart | Two 1 gram doses 12 hours apart | Two doses of 500 mg 12 hours apart | Single dose of 500 mg |
| Genital herpes: opening episode | 1 gram every 12 hours | No reduction | 1 gram every 24 hours | 500mg every 24 hours |
| Genital herpes: re-episode | 500 mg every 12 hours | No reduction | 500mg every 24 hours nine0110 | 500mg every 24 hours |
| Genital herpes: suppressive therapy |
|
|
|
|
| immunocompetent patients | 1g every 24 hours | No reduction | 500mg every 24 hours | 500mg every 24 hours |
| alternative doses for immunocompetent patients with ≤ 9 relapses/year | 500mg every 24 hours | No reduction | nine0613 500mg every 48 hours | 500mg every 48 hours |
| HIV-infected patients | 500mg every 12 hours | No reduction | 500mg every 24 hours | 500mg every 24 hours |
| 1 gram every 8 hours | 1 gram every 12 hours | 1 gram every 24 hours | 500mg every 24 hours | |
Hemodialysis: Patients requiring hemodialysis should receive the recommended doses of valaciclovir hydrochloride after hemodialysis. During hemodialysis, the half-life of acyclovir after taking valaciclovir hydrochloride is about 4 hours. About a third of acyclovir in the body is removed by dialysis during a 4-hour hemodialysis session. nine0035
During hemodialysis, the half-life of acyclovir after taking valaciclovir hydrochloride is about 4 hours. About a third of acyclovir in the body is removed by dialysis during a 4-hour hemodialysis session. nine0035
Peritoneal dialysis: There is no information available related to the use of valaciclovir hydrochloride in patients receiving peritoneal dialysis. The effect of chronic ambulatory peritoneal dialysis (CAPD) and continuous arteriovenous hemofiltration with concomitant hemodialysis (CAVHD) on the pharmacokinetics of acyclovir was studied. Elimination of aciclovir after CAPD and CAVHD is less pronounced than with hemodialysis, and pharmacokinetic parameters resemble those in patients with end-stage renal disease (ESRD) not receiving hemodialysis. Thus, there is no need for additional doses of valaciclovir hydrochloride in CAPD or CAVHD. nine0035
Thrombocytopenic acroangiothrombosis / hemolytic uremic syndrome sometimes fatal, observed in patients with HIV infection, as well as in allogeneic bone marrow and renal transplant recipients participating in clinical studies of valaciclovir hydrochloride at a dose of 8 g per day. Treatment with valaciclovir hydrochloride should be discontinued immediately upon observation of clinical signs, symptoms, and laboratory abnormalities consistent with thrombocytopenic acroangiothrombosis/hemolytic uremic syndrome. nine0035
Treatment with valaciclovir hydrochloride should be discontinued immediately upon observation of clinical signs, symptoms, and laboratory abnormalities consistent with thrombocytopenic acroangiothrombosis/hemolytic uremic syndrome. nine0035
Acute renal failure: Cases of acute renal failure have been reported in:
- elderly patients with or without decreased renal function. Caution should be exercised when prescribing valaciclovir hydrochloride to geriatric patients; dose reduction is recommended for people with impaired renal function.
- patients with underlying kidney disease who received valaciclovir hydrochloride above the recommended doses for their level of kidney function. In patients with renal insufficiency, a dose reduction is recommended when prescribing valaciclovir hydrochloride. nine0505
- patients receiving other nephrotoxic drugs. Caution should be exercised when prescribing valaciclovir hydrochloride to patients taking potentially nephrotoxic drugs.
- patients without adequate hydration. Precipitation of acyclovir in the renal tubules can be observed with increased solubility (2.5 mg / ml) in the intratubular fluid. Appropriate hydration should be maintained in all patients.
Central nervous system:
Adverse reactions of the central nervous system, including agitation, hallucinations, confusion, delirium, convulsions, and encephalopathy, have been reported in adult and pediatric patients with or without reduced renal function and in patients with comorbid conditions. kidney disease who received large recommended doses of valaciclovir hydrochloride for the level of their kidney function. Elderly patients are likely to have adverse reactions of the central nervous system. Valaciclovir hydrochloride should be discontinued if adverse reactions of the central nervous system occur. nine0035
Pediatric use: Efficacy and safety of valaciclovir have not been established in pediatric patients:
- Under 12 with herpes simplex;
- Under 18 with genital herpes;
- Under 18 years of age with herpes zoster;
- Under 2 years old and over 18 years old with chickenpox;
- In neonates on suppressive therapy after a herpes infection.
 nine0505
nine0505
Geriatric use:
Elderly patients experience decreased renal function and require dose reduction. Adverse reactions from the kidneys and the central nervous system are also more common in elderly patients.
Renal impairment:
When prescribing valaciclovir hydrochloride to patients with renal insufficiency, a dose reduction is recommended. nine0035
Use during pregnancy and lactation:
There have been no adequate and well-controlled studies of valacyclovir hydrochloride or acyclovir in pregnant women. Based on prospective pregnancy registry data in 749 pregnant women, the overall rate of birth defects in infants exposed to acyclovir in utero is the same as in infants in the general population. Valaciclovir hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. nine0035
A prospective epidemiological registry for the use of acyclovir during pregnancy was established in 1984 and completed in April 1999. It included 749 pregnant women exposed to systemic acyclovir during the first trimester of pregnancy, 756 results were recorded. The incidence of birth defects is approximately equal to that in the general population. However, the small size of the registry is not sufficient to assess risk for less common defects or to make reliable and definitive conclusions regarding the safety of acyclovir in pregnant women and their developing fetuses. nine0035
It included 749 pregnant women exposed to systemic acyclovir during the first trimester of pregnancy, 756 results were recorded. The incidence of birth defects is approximately equal to that in the general population. However, the small size of the registry is not sufficient to assess risk for less common defects or to make reliable and definitive conclusions regarding the safety of acyclovir in pregnant women and their developing fetuses. nine0035
No teratogenic effects have been found in animal studies.
Aciclovir, the main metabolite of valaciclovir, is excreted in breast milk. After the appointment of valaciclovir orally at a dose of 500 mg C m ax acyclovir in breast milk was 0.5-2.3 times (on average 1.4 times) higher than the corresponding concentrations in the mother's blood. The ratio of the AUC of acyclovir found in breast milk to the AUC of acyclovir in maternal plasma was 1.4-2.6 (mean 2.2). The average concentration of acyclovir in breast milk is 2. 24 μg / ml. When a nursing mother is given valaciclovir 500 mg twice daily, the child will be exposed to the same effects of aciclovir as when taken orally at a dose of about 0.61 mg/kg/day. Valaciclovir unchanged is not detected in the blood and breast milk of the mother or the urine of the child. Valaciclovir should be administered to nursing women with caution, only if necessary. nine0035
24 μg / ml. When a nursing mother is given valaciclovir 500 mg twice daily, the child will be exposed to the same effects of aciclovir as when taken orally at a dose of about 0.61 mg/kg/day. Valaciclovir unchanged is not detected in the blood and breast milk of the mother or the urine of the child. Valaciclovir should be administered to nursing women with caution, only if necessary. nine0035
Influence on the ability to drive vehicles and other potentially dangerous mechanisms:
The drug does not affect the ability to drive vehicles and engage in activities that require high concentration of attention.
The following adverse reactions have been identified in clinical trials:
In adults:
Valaciclovir tablets. nine0035
Nervous side effects include aggressive behavior, unsteady movements, unsteady gait, confusion, speech, hallucinations, convulsions. Kidney problems and nervous system problems are seen in patients who already have kidney disease, as well as in elderly patients whose kidney function is reduced due to age. Common adverse reactions in adults include: headache, nausea, abdominal pain, vomiting and dizziness. Adverse reactions in HIV-infected adults: headache, fatigue and rash. These adverse reactions are usually mild and do not force patients to stop taking Valaciclovir tablets. nine0035
Common adverse reactions in adults include: headache, nausea, abdominal pain, vomiting and dizziness. Adverse reactions in HIV-infected adults: headache, fatigue and rash. These adverse reactions are usually mild and do not force patients to stop taking Valaciclovir tablets. nine0035
Other, less common, adverse reactions in adults include painful menses in women, joint pain, depression, elevated alkaline phosphatase, elevated ALT, AST, decreased neutrophils and decreased platelets, nasopharyngitis, and upper respiratory tract infections.
Pediatric patients at aged 12 to 18 years: headache, nausea.
Pediatric patients ages 1 month to 12 years: diarrhoea, pyrexia, dehydration, herpes simplex virus and runny nose. There were no clinically significant changes in laboratory parameters.
In addition, the following adverse reactions have been observed during post-marketing use of valaciclovir hydrochloride:
Allergic reactions: acute hypersensitivity reactions, including anaphylactic shock, angioedema, dyspnea, pruritus, rash and urticaria
From the side of the central nervous system : aggressive behavior, agitation, ataxia, coma, confusion; clouding of consciousness, dysarthria, encephalopathy, mania and psychosis, including auditory and visual hallucinations, convulsions, tremor.
On the part of the organ of vision: visual disturbances.
From the gastrointestinal tract and hepatobiliary system: diarrhea, abnormal liver enzymes, hepatitis.
From the urinary system: renal insufficiency, renal pain (may be associated with renal insufficiency).
Hematological parameters: thrombocytopenia, aplastic anemia, leukocytoclastic vasculitis, thrombocytopenic acroangiothrombosis / hemolytic uremic syndrome.
Skin: erythema, rash including photosensitivity, alopecia.
Other: Facial edema, hypertension, tachycardia. nine0035
Contraindications
Valaciclovir hydrochloride is contraindicated in patients with clinically significant hypersensitivity reactions (eg, anaphylaxis) to valaciclovir, aciclovir, or any component of the drug.
Overdose
Care must be taken to prevent accidental overdose. Precipitation of aciclovir in the renal tubules may be observed due to increased solubility (2.
 83 (±0.14)
83 (±0.14)  03 (±1.37)
03 (±1.37)