Most common side effects of citalopram
Side effects of citalopram - NHS
Like all medicines, citalopram can cause side effects in some people, but many people have no side effects.
Some of the common side effects of citalopram will gradually improve as your body gets used to it.
For example, taking citalopram for panic attacks can sometimes make anxiety worse during the first few weeks of treatment. But this usually wears off after a few weeks. If it bothers you, speak to your doctor about it. A lower dose may help reduce your symptoms.
Common side effects
These common side effects of citalopram happen in more than 1 in 100 people. There are things you can do to help cope with them:
Dry mouthChew sugar-free gum or suck sugar-free sweets.
Try wearing loose clothing, use a strong anti-perspirant and keep cool using a fan if possible. If this does not help, you may need to try a different type of antidepressant.
Being unable to sleepTake citalopram first thing in the morning.
Feeling sleepy, tired or weakTake citalopram in the evening and try to drink less alcohol. Do not drive, ride a bike or use tools or machinery if you're feeling sleepy. If this does not help, talk to your doctor.
Feeling tired or weakDo not drive, ride a bike or use tools or machinery if you're feeling tired. Cut down the amount of alcohol you drink as it can make you feel worse.
HeadachesMake sure you rest and drink plenty of fluids. Do not drink too much alcohol. Ask your pharmacist to recommend a painkiller. Talk to your doctor if the headaches do not go away or are severe.
Talk to your doctor if the headaches do not go away or are severe.
Try taking citalopram with or after food. It may also help if you avoid rich or spicy food. If it carries on, tell your doctor.
Speak to a doctor or pharmacist if the advice on how to cope does not help and a side effect is still bothering you or does not go away.
Serious side effects
Serious side effects are rare and happen in less than 1 in 1,000 people.
Book an appointment with your doctor if:
- you have changes in your periods, such as heavy bleeding, spotting or bleeding between periods
- you gain or lose weight without trying
Call a doctor or contact 111 straight away if you:
- start bleeding from the gums or get bruises that appear without a reason or that get bigger
- have strong feelings of happiness, enthusiasm or excitement, or feeling restless and you cannot sit or stand still
- start coughing up blood or have blood in your pee
- have black or red poo, or blood in your vomit – these can be signs of bleeding from the gut
Go to 111. nhs.uk or call 111.
nhs.uk or call 111.
Immediate action required: Call 999 or go to A&E now if:
- you have chest pain or pressure or shortness of breath
- you have a fit or seizure for the first time, or the seizures you have had in the past become more frequent
- you feel very dizzy, or pass out
- you have painful erections that last longer than 2 hours – this may happen even when you're not having sex
- you get bleeding from any cuts or nosebleeds that is very heavy or does not stop within 10 minutes
- you have thoughts about harming yourself or ending your life
- you get headaches, have trouble focusing, have memory problems, cannot think clearly, have weakness, have a seizure or fit, or lose your balance – these can be signs of low sodium levels
Find your nearest A&E
Serious allergic reaction
In rare cases, it's possible to have a serious allergic reaction (anaphylaxis) to citalopram.
Immediate action required: Call 999 or go to A&E now if:
- you get a skin rash that may include itchy, red, swollen, blistered or peeling skin
- you're wheezing
- you get tightness in the chest or throat
- you have trouble breathing or talking
- your mouth, face, lips, tongue or throat start swelling
You could be having a serious allergic reaction and may need immediate treatment in hospital.
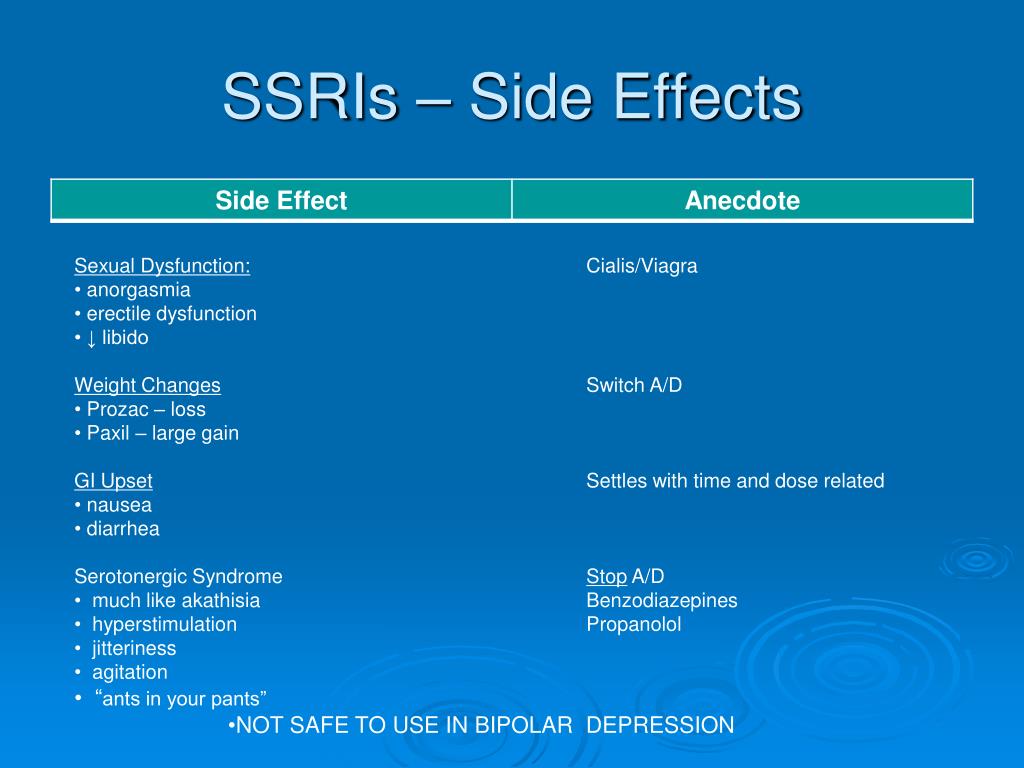
Sexual side effects
The good effects of citalopram may, after a while, improve your sex life as your mood lifts and you start to feel like yourself again.
Some of the possible sexual side effects include:
- painful erections and problems getting an erection and ejaculating
- some vaginal bleeding
- not reaching orgasm the same way as before
- a lower sex drive
Sexual side effects usually stop after the first couple of weeks. Sometimes, however, they can be long lasting and may not get better even after you stop taking the medicine. If this happens and it’s a problem for you, go back to your doctor to see if there's another treatment you can try.
Sometimes, however, they can be long lasting and may not get better even after you stop taking the medicine. If this happens and it’s a problem for you, go back to your doctor to see if there's another treatment you can try.
Other side effects
These are not all the side effects of citalopram. For a full list see the leaflet inside your medicines packet.
Information:
You can report any suspected side effect using the Yellow Card safety scheme.
Visit Yellow Card for further information.
Page last reviewed: 9 February 2022
Next review due: 9 February 2025
Citalopram Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing
Warnings:
Antidepressant medications are used to treat a variety of conditions, including depression and other mental/mood disorders. These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, studies have shown that a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, studies have shown that a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
Tell the doctor right away if you notice worsening depression/other psychiatric conditions, unusual behavior changes (including possible suicidal thoughts/attempts), or other mental/mood changes (including new/worsening anxiety, panic attacks, trouble sleeping, irritability, hostile/angry feelings, impulsive actions, severe restlessness, very rapid speech). Be especially watchful for these symptoms when a new antidepressant is started or when the dose is changed.
Warnings:
Antidepressant medications are used to treat a variety of conditions, including depression and other mental/mood disorders. These medications can help prevent suicidal thoughts/attempts and provide other important benefits. However, studies have shown that a small number of people (especially people younger than 25) who take antidepressants for any condition may experience worsening depression, other mental/mood symptoms, or suicidal thoughts/attempts. It is very important to talk with the doctor about the risks and benefits of antidepressant medication (especially for people younger than 25), even if treatment is not for a mental/mood condition.
Tell the doctor right away if you notice worsening depression/other psychiatric conditions, unusual behavior changes (including possible suicidal thoughts/attempts), or other mental/mood changes (including new/worsening anxiety, panic attacks, trouble sleeping, irritability, hostile/angry feelings, impulsive actions, severe restlessness, very rapid speech). Be especially watchful for these symptoms when a new antidepressant is started or when the dose is changed.
Be especially watchful for these symptoms when a new antidepressant is started or when the dose is changed.
... Show More
Uses
Citalopram is used to treat depression. It may improve your energy level and feelings of well-being. Citalopram is known as a selective serotonin reuptake inhibitor (SSRI). This medication works by helping to restore the balance of a certain natural substance (serotonin) in the brain.
How to use Citalopram HBR
Read the Medication Guide and, if available, the Patient Information Leaflet provided by your pharmacist before you start taking citalopram and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
Take this medication with or without food as directed by your doctor, usually once daily in the morning or evening. The dosage is based on your medical condition, response to treatment, age, laboratory tests, and other medications you may be taking. Be sure to tell your doctor and pharmacist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
Be sure to tell your doctor and pharmacist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
If you are using the liquid form of this medication, carefully measure the dose using a special measuring device/spoon. Do not use a household spoon because you may not get the correct dose.
To reduce your risk of side effects, your doctor may direct you to start taking this drug at a low dose and gradually increase your dose. Follow your doctor's instructions carefully. Do not increase your dose or use this drug more often or for longer than prescribed. Your condition will not improve any faster, and your risk of side effects will increase. Take this medication regularly to get the most benefit from it. To help you remember, take it at the same time each day.
Keep taking this medication even if you feel well. Do not stop taking this medication without consulting your doctor. Some conditions may become worse when this drug is suddenly stopped. Also, you may experience symptoms such as mood swings, headache, tiredness, sleep changes, and brief feelings similar to electric shock. To prevent these symptoms while you are stopping treatment with this drug, your doctor may reduce your dose gradually. Consult your doctor or pharmacist for more details. Report any new or worsening symptoms right away.
Also, you may experience symptoms such as mood swings, headache, tiredness, sleep changes, and brief feelings similar to electric shock. To prevent these symptoms while you are stopping treatment with this drug, your doctor may reduce your dose gradually. Consult your doctor or pharmacist for more details. Report any new or worsening symptoms right away.
It may take 1 to 4 weeks to feel a benefit from this drug and up to several weeks before you get the full benefit.
Tell your doctor if your condition does not improve or if it worsens.
Side Effects
See also Warning and Precautions sections.
Nausea, dry mouth, loss of appetite, tiredness, drowsiness, sweating, blurred vision, and yawning may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.
Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
Tell your doctor right away if you have any serious side effects, including: shaking (tremor), decreased interest in sex, changes in sexual ability, easy bruising/bleeding.
Get medical help right away if you have any very serious side effects, including: fainting, fast/irregular heartbeat, black stools, vomit that looks like coffee grounds, seizures, eye pain/swelling/redness, widened pupils, vision changes (such as seeing rainbows around lights at night).
This medication may increase serotonin and rarely cause a very serious condition called serotonin syndrome/toxicity. The risk increases if you are also taking other drugs that increase serotonin, so tell your doctor or pharmacist of all the drugs you take (see Drug Interactions section). Get medical help right away if you develop some of the following symptoms: fast heartbeat, hallucinations, loss of coordination, severe dizziness, severe nausea/vomiting/diarrhea, twitching muscles, unexplained fever, unusual agitation/restlessness.
Rarely, males may have a painful or prolonged erection lasting 4 or more hours. If this occurs, stop using this drug and get medical help right away, or permanent problems could occur.
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US - Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
Precautions
Before taking citalopram, tell your doctor or pharmacist if you are allergic to it; or to escitalopram; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: personal or family history of bipolar/manic-depressive disorder, personal or family history of suicide attempts, liver disease, seizures, low sodium in the blood, intestinal ulcers/bleeding (peptic ulcer disease) or bleeding problems, personal or family history of glaucoma (angle-closure type).
Citalopram may cause a condition that affects the heart rhythm (QT prolongation). QT prolongation can rarely cause serious (rarely fatal) fast/irregular heartbeat and other symptoms (such as severe dizziness, fainting) that need medical attention right away.
The risk of QT prolongation may be increased if you have certain medical conditions or are taking other drugs that may cause QT prolongation. Before using citalopram, tell your doctor or pharmacist of all the drugs you take and if you have any of the following conditions: certain heart problems (heart failure, slow heartbeat, recent heart attack, QT prolongation in the EKG), family history of certain heart problems (QT prolongation in the EKG, sudden cardiac death).
Low levels of potassium or magnesium in the blood may also increase your risk of QT prolongation. This risk may increase if you use certain drugs (such as diuretics/"water pills") or if you have conditions such as severe sweating, diarrhea, or vomiting. Talk to your doctor about using citalopram safely.
This drug may make you drowsy or blur your vision. Alcohol or marijuana (cannabis) can make you more drowsy. Do not drive, use machinery, or do anything that needs alertness or clear vision until you can do it safely. Avoid alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).
Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
Older adults may be more sensitive to the side effects of this drug, especially bleeding, loss of coordination, and QT prolongation (see above). They may also be more likely to develop a type of salt imbalance (hyponatremia), especially if they are also taking "water pills" (diuretics).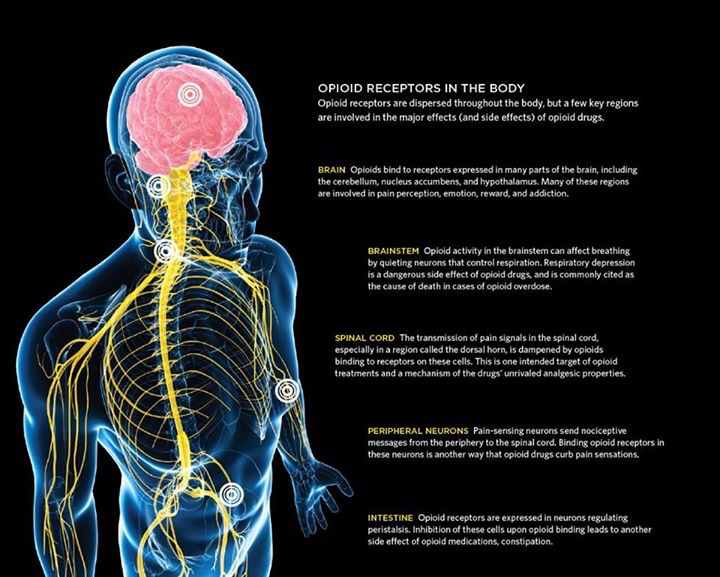 Loss of coordination can increase the risk of falling.
Loss of coordination can increase the risk of falling.
Children may be more sensitive to the side effects of this drug, especially loss of appetite and weight loss. Monitor weight and height in children who are taking this drug.
During pregnancy, this medication should be used only when clearly needed. It may harm an unborn baby. Also, babies born to mothers who have used this drug during the last 3 months of pregnancy may rarely develop withdrawal symptoms such as feeding/breathing difficulties, seizures, muscle stiffness, or constant crying. If you notice any of these symptoms in your newborn, tell the doctor promptly.
Since untreated mental/mood problems (such as depression, obsessive-compulsive disorder, panic disorder) can be a serious condition, do not stop taking this medication unless directed by your doctor. If you are planning pregnancy, become pregnant, or think you may be pregnant, immediately discuss with your doctor the benefits and risks of using this medication during pregnancy.
This drug passes into breast milk and may have undesirable effects on a nursing infant. Consult your doctor before breast-feeding.
Interactions
See also Precautions section.
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Some products that may interact with this drug include: other drugs that can cause bleeding/bruising (including antiplatelet drugs such as clopidogrel, NSAIDs such as ibuprofen, "blood thinners" such as warfarin).
Aspirin can increase the risk of bleeding when used with this medication. However, if your doctor has directed you to take low-dose aspirin for heart attack or stroke prevention (usually 81-162 milligrams a day), you should continue taking it unless your doctor instructs you otherwise. Ask your doctor or pharmacist for more details.
Ask your doctor or pharmacist for more details.
Taking MAO inhibitors with this medication may cause a serious (possibly fatal) drug interaction. Avoid taking MAO inhibitors (isocarboxazid, linezolid, metaxalone, methylene blue, moclobemide, phenelzine, procarbazine, rasagiline, safinamide, selegiline, tranylcypromine) during treatment with this medication. Most MAO inhibitors should also not be taken for two weeks before and after treatment with this medication. Ask your doctor when to start or stop taking this medication.
The risk of serotonin syndrome/toxicity increases if you are also taking other drugs that increase serotonin. Examples include street drugs such as MDMA/"ecstasy," St. John's wort, certain antidepressants (including other SSRIs such as fluoxetine/paroxetine, SNRIs such as duloxetine/venlafaxine), tryptophan, among others. The risk of serotonin syndrome/toxicity may be more likely when you start or increase the dose of these drugs.
Tell your doctor or pharmacist if you are taking other products that cause drowsiness including alcohol, marijuana (cannabis), antihistamines (such as cetirizine, diphenhydramine), drugs for sleep or anxiety (such as alprazolam, diazepam, zolpidem), muscle relaxants, and opioid pain relievers (such as codeine).
Check the labels on all your medicines (such as allergy or cough-and-cold products) because they may contain ingredients that cause drowsiness. Ask your pharmacist about using those products safely.
Many drugs besides citalopram may affect the heart rhythm (QT prolongation), including amiodarone, pimozide, procainamide, quinidine, sotalol, among others.
Citalopram is very similar to escitalopram. Do not use medications containing escitalopram while using citalopram.
This medication may interfere with certain medical/laboratory tests (including brain scan for Parkinson's disease), possibly causing false test results. Make sure laboratory personnel and all your doctors know you use this drug.
Does Citalopram HBR interact with other drugs you are taking?
Enter your medication into the WebMD interaction checker
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center.
Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center.
Do not share this medication with others.
Laboratory and/or medical tests (such as EKG) should be done periodically to monitor your progress or check for side effects. Consult your doctor for more details.
Keep all regular medical and psychiatric appointments.
If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Images
Next
Related Links
Drug Survey
Are you currently using Citalopram HBR?
This survey is being conducted by the WebMD marketing sciences department.
Free RX Coupon
Save up to 80% on your prescriptions.
Available coupons
Save up to 80% on your prescription with WebMDRx
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.
A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.
SIDE EFFECTS OF ANTIDEPRESSANTS (SSRIs) | Clinical Center "Psychiatry - Narcology"
Currently, the most commonly prescribed antidepressants are drugs from the group of selective serotonin reuptake inhibitors (SSRIs). For most, these medicines are safe and effective, but like all medicines, they can cause side effects. According to statistics, about 40% of patients taking antidepressants also experience side effects, in about 25% of cases they are quite unpleasant. Two of the most common side effects (sexual dysfunction and weight gain) are often the reason people stop taking these medications.
Listed below are the 7 most common side effects of antidepressants that patients should be aware of:
1. SOMATIC SYMPTOMS.
When medications are first prescribed to treat depression, the most common physical symptoms are headache, nausea, joint and muscle pain, rash, and diarrhea. These symptoms are usually mild and temporary. The results of clinical studies have shown that nausea and headache are the most common. As a rule, these symptoms are adaptive in nature, as a rule, they pass on their own, without requiring discontinuation of the drug.
These symptoms are usually mild and temporary. The results of clinical studies have shown that nausea and headache are the most common. As a rule, these symptoms are adaptive in nature, as a rule, they pass on their own, without requiring discontinuation of the drug.
2. SLEEP DISTURBANCE.
Many patients, when first prescribed antidepressants, report problems with sleep: difficulty falling asleep or light sleep with frequent awakenings. Also, against the background of taking SSRIs, nightmares and sleepwalking can be observed. Studies have shown that about 22% of people taking antidepressants experience sleep problems.
3. DAY SLEEPNESS .
Sleepiness during the day may be the result of a poor night's sleep, or the direct sedative effect of the antidepressant. In the case when it is a sedative effect, the problem can be solved by transferring the drug to the evening.
4. MIGRAINES
Due to the fact that people who are prone to depression also often suffer from migraines, you need to be careful when taking medications in combination. Medicines; used to treat migraines, triptans, like SSRIs, increase serotonin levels in the brain. If these drugs are used together, it can lead to the development of serotonin syndrome, which manifests itself in the form of headache, heart palpitations, hot flashes. Be sure to discuss with your doctor how to avoid the development of serotonin syndrome if you are prescribed medications of both groups.
Medicines; used to treat migraines, triptans, like SSRIs, increase serotonin levels in the brain. If these drugs are used together, it can lead to the development of serotonin syndrome, which manifests itself in the form of headache, heart palpitations, hot flashes. Be sure to discuss with your doctor how to avoid the development of serotonin syndrome if you are prescribed medications of both groups.
5. WEIGHT SET.
Weight gain is one of the late side effects of antidepressants and is one of the most common reasons for refusing to continue taking or changing the drug. A good prevention of this side effect is moderate physical activity (for example, a 30-minute workout every other day). The likelihood of weight gain also depends on the drug that is prescribed. According to clinical trials, while taking paroxetine, about 25% of patients gain 7% of their weight.
6. SUICIDE .
The risk of suicide while taking antidepressants is currently under extensive investigation. According to most studies, compared with placebo, taking SSRIs or other antidepressants doubles the likelihood of suicidal thoughts. The overall risk of this side effect when taking antidepressants in adolescents and adults is 2 to 4 percent. One of the reasons for suicide while taking antidepressants is that medications increase activity, giving energy for the implementation of a suicidal plan. Regular follow-up by a doctor can reduce the risk of this side effect.
According to most studies, compared with placebo, taking SSRIs or other antidepressants doubles the likelihood of suicidal thoughts. The overall risk of this side effect when taking antidepressants in adolescents and adults is 2 to 4 percent. One of the reasons for suicide while taking antidepressants is that medications increase activity, giving energy for the implementation of a suicidal plan. Regular follow-up by a doctor can reduce the risk of this side effect.
7. SEXUAL DYSFUNCTION.
Sexual dysfunction is one of the most common long-term side effects of SSRIs. These include decreased sexual desire, delayed ejaculation in men, and inability to achieve orgasm in women. Up to 60% of people taking SSRIs experience one of these side effects. And these are the side effects that patients are not ready to endure.
If you are concerned about any side effects, discuss them with your doctor. As a rule, you can find a solution for any of them. It is not recommended to stop taking medications on your own.
Citalopram: use in elderly patients (extended abstract) - Psychiatry and psychopharmacotherapy. P.B. Gannushkina №05 2001
90 0 3
Subscribe to new numbers
Author: C.-G. Gottfries, B. G. Pollock
Gothenburg, Sweden
Issue page numbers: 172-175
The chemical structure of citalopram, a derivative of bicyclic isobenzofuran, has no analogues among other antidepressants. The potent and most selective SSRI currently available, citalopram, has virtually no effect on the reuptake of norepinephrine, dopamine and GABA, as well as on the receptors of other neurotransmitters.
The prevalence of depressive disorders in the elderly is estimated at 12–15% [1]. The Geriatric Psychiatric Union estimates that the number of patients with depression in this age group in the United States is approaching 6 million [2].
In a survey of 1,070 randomly selected individuals aged 65 years and over living in Liverpool, England, it was found that the prevalence of clinical depression was 11.3%; in another 10.7% of cases, subsyndromal disorders were noted [3]. Major depression in elderly patients is less common than secondary or minor depression. The rate of minor depression has been reported to be as high as 50% [1, 4, 5].
The likelihood of early relapse after the first depressive episode increases with age [6, 7]. The likelihood of complete recovery after an episode of major depression in the elderly is reduced, and residual symptoms may persist even with a decrease in their severity [8].
According to available data, 40-50% of patients with Alzheimer's disease have signs of low mood, and 10-20% have clinically significant current depressive disorders [9]. Reifler et al. [10] found comorbid depression in about 25% of 103 geriatric outpatients with dementia (according to DSM-III). In a subsequent study by Reifler et al. [11] found that 31% of 131 patients (mean age 77 years) with Alzheimer's dementia met the DSM-III criteria for major depressive disorder at baseline. Elderly patients with certain somatic diseases, such as cancer, CNS vascular disease, and Parkinson's disease, show a higher prevalence of major and minor depression than other persons of the considered age [12-14].
[11] found that 31% of 131 patients (mean age 77 years) with Alzheimer's dementia met the DSM-III criteria for major depressive disorder at baseline. Elderly patients with certain somatic diseases, such as cancer, CNS vascular disease, and Parkinson's disease, show a higher prevalence of major and minor depression than other persons of the considered age [12-14].
Unfortunately, the use of antidepressants in this age group has only been studied in a few controlled clinical trials. As a result, guidelines for the use of antidepressants in the elderly extrapolate data originally obtained from patients with depression in adolescence and middle age [16].
Inadequate detection, misdiagnosis and treatment of depression in the elderly is the rule rather than the exception [2, 17, 18]. One of the reasons for this lies in the fact that depression is often masked or provoked by comorbid somatic diseases, drugs or alcohol [1]. For example, depression is often viewed in older patients with cognitive impairments [1, 2, 10]. It is possible that such patients simply cannot give accurate anamnestic information about themselves [18]. Elderly patients with depression are likely to have difficulty recognizing and describing their own depressive symptoms [19], while they may fear that they will be diagnosed with dementia if they complain of slow thinking or memory impairment. In general, they are more likely to report somatic than psychiatric symptoms [17, 18]. Elderly patients, their family members, and even some clinicians may view depression as a normal part of the aging process or a normal response to the many losses associated with old age (eg, death of loved ones, loss of a job).
It is possible that such patients simply cannot give accurate anamnestic information about themselves [18]. Elderly patients with depression are likely to have difficulty recognizing and describing their own depressive symptoms [19], while they may fear that they will be diagnosed with dementia if they complain of slow thinking or memory impairment. In general, they are more likely to report somatic than psychiatric symptoms [17, 18]. Elderly patients, their family members, and even some clinicians may view depression as a normal part of the aging process or a normal response to the many losses associated with old age (eg, death of loved ones, loss of a job).
Currently, the use of selective serotonin reuptake inhibitors (SSRIs) in the treatment of depression in the elderly is growing significantly. This is because this class of antidepressants causes fewer of the adverse and sometimes dangerous side effects associated with tricyclic antidepressants (TCAs), including anticholinergic-related urinary retention, constipation, confusion, as well as cardiotoxicity, orthostatic hypotension, and potential mortality. in case of overdose [19]. Among SSRIs, citalopram is a particularly attractive antidepressant for older depressed patients. It was first approved in Denmark in 1989 and is currently one of the most widely used antidepressants in most European countries and the USA.
in case of overdose [19]. Among SSRIs, citalopram is a particularly attractive antidepressant for older depressed patients. It was first approved in Denmark in 1989 and is currently one of the most widely used antidepressants in most European countries and the USA.
This publication is a review of studies on the pharmacological profile, efficacy, safety and tolerability of citalopram, as well as several studies specifically on the use of this antidepressant in elderly patients with depression.
Pharmacodynamic and pharmacokinetic features
The chemical structure of citalopram, a bicyclic isobenzofuran derivative, is unique among other antidepressants. The potent and most selective SSRI currently available, citalopram, has virtually no effect on the reuptake of norepinephrine, dopamine and GABA, as well as on the receptors of other neurotransmitters. Since the main metabolites of citalopram have the same selectivity and are found in blood plasma in low concentrations, they practically do not affect the biochemical activity of citalopram [22, 23, 27, 28].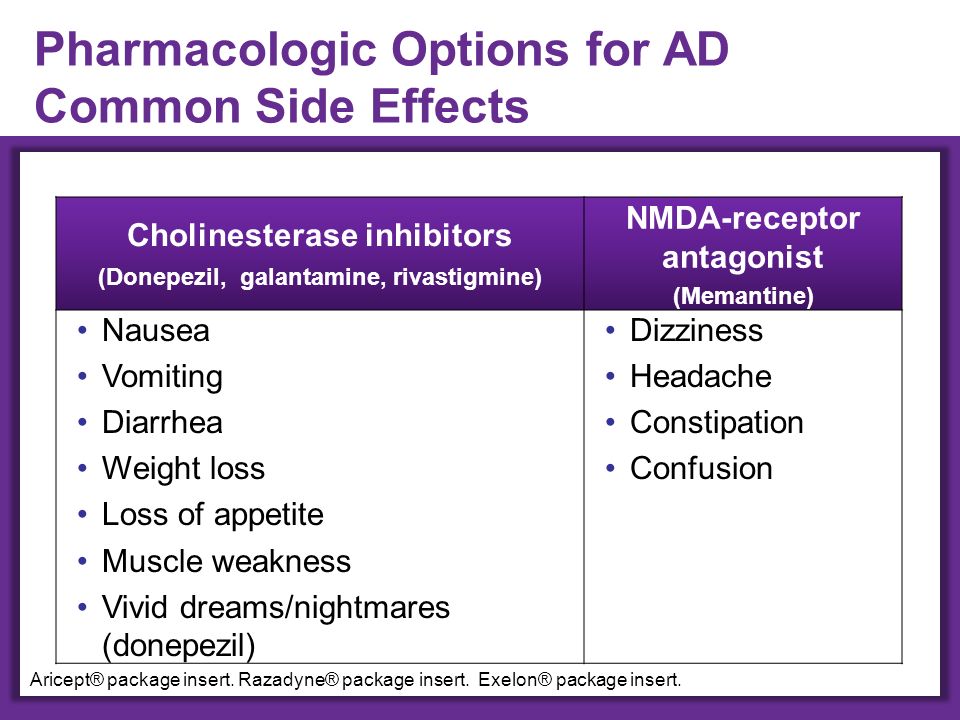
The weak affinity of citalopram to other receptors (histamine, acetylcholine, dopamine, alpha-adrenergic receptors, etc.) and enzymatic systems distinguishes this drug from TCAs and, to a lesser extent, from other SSRIs [25]. This feature explains why the considered antidepressant practically does not cause anticholinergic and cardiotoxic side effects characteristic of TCAs [23].
The results of a study of the pharmacokinetics of citalopram in 8 healthy subjects aged 23 to 34 years demonstrate that the antidepressant is absorbed quickly and almost completely, regardless of food intake. The high bioavailability (mean 80%) of citalopram indicates that the first pass metabolism of the drug is limited. After oral administration of 50 mg of an antidepressant to patients, its maximum concentration in blood plasma was reached after 2-4 hours. In most patients, with regular oral administration of the drug, its stable concentration in blood plasma was established within 1-2 weeks. This concentration was linearly related to the dosage used [27, 32]. Such a direct relationship reduces the risk of potentially disproportionate changes in the properties of the antidepressant associated with fluctuations in its concentration, which in turn may be due to inhibition of serotonin reuptake or interaction with specific enzymes - cytochromes P-450 (CTX) [33].
This concentration was linearly related to the dosage used [27, 32]. Such a direct relationship reduces the risk of potentially disproportionate changes in the properties of the antidepressant associated with fluctuations in its concentration, which in turn may be due to inhibition of serotonin reuptake or interaction with specific enzymes - cytochromes P-450 (CTX) [33].
Efficacy in elderly patients
Although data on the effectiveness of citalopram and other SSRIs in the treatment of depression and other emotional disorders in young patients is very extensive, data on the clinical activity of these antidepressants in the elderly is limited [34]. In the Scandinavian study by Nyth and Gottfries [35], performed in 1990, researchers used a combined method (double-blind and open-label) to compare the results of treatment with citalopram and placebo for symptoms of dementia in elderly patients. This study was not designed specifically to assess the effectiveness of SSRIs for depression, but results from initial testing of patients using the Montgomery-Asberg Depression Rating Scale (MADRS) indicate that most of those surveyed had symptoms of mild depression.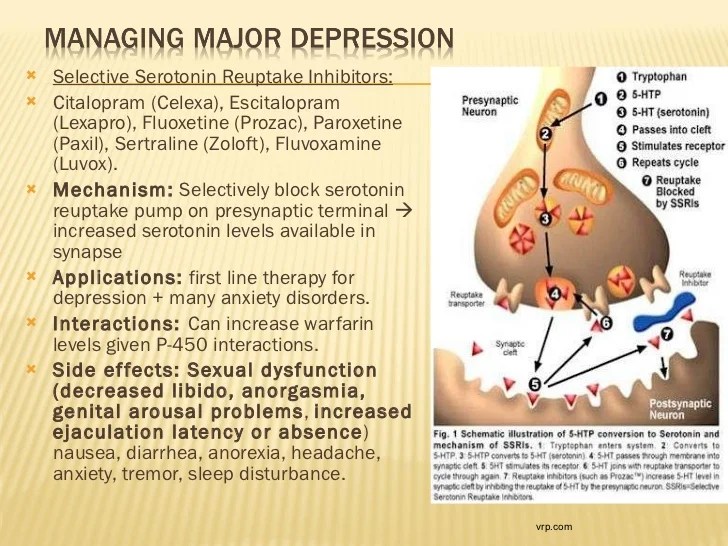 Studied the effectiveness of treatment 89patients (mean age 77.6 years) with moderate Alzheimer's disease, senile dementia of the Alzheimer's type or vascular dementia. Patients with severe dementia were not included in the study. Psychiatric severity was assessed using the Global Clinical Impression (CGI) scale, and the severity of worsening or improving symptoms was assessed using the Gottfries-Brane-Steen Geriatric Rating Scale (GBS) and MADRS. Overall, the results suggest that, after a 4-week course of treatment in patients with Alzheimer's disease or Alzheimer's-type senile dementia, citalopram (n=31) was significantly more effective than placebo (n=45) in reducing low mood (p<0.01) and other emotional disorders (emotional dullness, confusion, irritability, anxiety, fear-panic, restlessness). Patients with vascular dementia (n=13) showed no significant improvement.
Studied the effectiveness of treatment 89patients (mean age 77.6 years) with moderate Alzheimer's disease, senile dementia of the Alzheimer's type or vascular dementia. Patients with severe dementia were not included in the study. Psychiatric severity was assessed using the Global Clinical Impression (CGI) scale, and the severity of worsening or improving symptoms was assessed using the Gottfries-Brane-Steen Geriatric Rating Scale (GBS) and MADRS. Overall, the results suggest that, after a 4-week course of treatment in patients with Alzheimer's disease or Alzheimer's-type senile dementia, citalopram (n=31) was significantly more effective than placebo (n=45) in reducing low mood (p<0.01) and other emotional disorders (emotional dullness, confusion, irritability, anxiety, fear-panic, restlessness). Patients with vascular dementia (n=13) showed no significant improvement.
In 1992, Nyth et al. [36] compared the effectiveness of citalopram at doses of 20 and 30 mg per day with placebo in the treatment of emotional disorders in patients with a clinical diagnosis of depression, whose age ranged from 65 to 91 years. In this 6-week, double-blind, multicenter study in Scandinavia, 98 (74%) of 133 patients had major depression and 29 (22%) also had dementia. Improvement on the background of citalopram (n=88) was more pronounced than in the control group (n=45). Differences in the average total score of the Hamilton depression scale (HDRS) and the degree of its reduction were statistically significant (p<0.05 and p<0.01, respectively). The proportion of responders (according to MADRS) among those treated with antidepressant and placebo was 53% and 28%, respectively (p<0.05). According to CGI estimates, 60% of patients treated with citalopram improved "very significantly" or "significantly". The same figure among patients taking placebo was only 24% (p<0.001). Changes in the GBS scale in the subgroup of patients with concomitant dementia indicated that some intellectual and emotional functions also improved, such as time orientation, short-term memory. The severity of anxiety and fear-panic also decreased.
In this 6-week, double-blind, multicenter study in Scandinavia, 98 (74%) of 133 patients had major depression and 29 (22%) also had dementia. Improvement on the background of citalopram (n=88) was more pronounced than in the control group (n=45). Differences in the average total score of the Hamilton depression scale (HDRS) and the degree of its reduction were statistically significant (p<0.05 and p<0.01, respectively). The proportion of responders (according to MADRS) among those treated with antidepressant and placebo was 53% and 28%, respectively (p<0.05). According to CGI estimates, 60% of patients treated with citalopram improved "very significantly" or "significantly". The same figure among patients taking placebo was only 24% (p<0.001). Changes in the GBS scale in the subgroup of patients with concomitant dementia indicated that some intellectual and emotional functions also improved, such as time orientation, short-term memory. The severity of anxiety and fear-panic also decreased.
Based on a review of these two studies, Gottfries et al. [37] concluded that the use of citalopram may be useful in the treatment of not only depression, but other emotional disorders.
Efficacy in post-stroke depression
The efficacy and safety of citalopram in the treatment of 66 patients with post-stroke depression was studied in a randomized, placebo-controlled, double-blind study [38]. The mean age of the 33 examined patients who received citalopram was 68 years, and those who received placebo (n=33) was 66 years. The HDRS-13 total score was used for inclusion in the study. Significantly greater improvement (p<0.05) in HDRS was observed in patients who received citalopram (10 to 40 mg daily). Efficacy was assessed by the change in the initial HDRS score [39].
There were very few side effects, they were all mild and transient. Nausea and vomiting were more common during the 1st week of treatment in the citalopram group (p<0. 05). No sedation or confusion was observed. The researchers concluded that citalopram is an effective drug for the treatment of post-stroke depression, especially if the depression begins more than 8 weeks after a stroke, when spontaneous recovery is unlikely. Therapy with citalopram is well tolerated by patients.
05). No sedation or confusion was observed. The researchers concluded that citalopram is an effective drug for the treatment of post-stroke depression, especially if the depression begins more than 8 weeks after a stroke, when spontaneous recovery is unlikely. Therapy with citalopram is well tolerated by patients.
Efficacy in emotional and behavioral complications of dementia
Data from a limited number of sources indicate that citalopram has a beneficial effect on behavioral disorders observed in old age, such as disinhibition, agitation, hostility, confusion, and anxiety. As previously discussed in a study by Nyth and Gottfries [35], citalopram significantly contributes to the reduction of behavioral disorders in elderly patients with moderate dementia. In a study by Nyth et al. [36] also obtained data indicating that in elderly patients with dementia and a clinically diagnosed depression treated with citalopram, there was a significant reduction in anxiety and fear-panic, documented by the GBS scale (p<0. 01, paired t-test ).
01, paired t-test ).
Data from an open 12-month study performed in Sweden also indicate that the antidepressant under consideration is effective in the treatment of emotional disorders that manifest in old age [40]. These results were obtained in a survey of 123 patients (mean age 78 years), 76% of whom at the time of inclusion in the sample had dementia with associated "emotional symptoms" such as low mood and irritability (by GBS). Citalopram resulted in significant clinical improvement in 60% of patients, including a positive effect on depression and irritability after 1 month of treatment, anxiety and fear-panic after 3 months. Citalopram had no effect on cognitive functions.
The efficacy of citalopram in the treatment of behavioral complications of dementia was also studied in 16 inpatients aged 60–99 years (mean age 77.6 years) [41]. Patients in this open-label pilot study showed signs of severe agitation and severe psychotic symptoms. Completed a 17-day course of therapy 13 patients. Agitation decreased by 44.7% and hostility by 52.5%.
Agitation decreased by 44.7% and hostility by 52.5%.
Tolerability and safety
The degree of tolerability of citalopram was determined using an integrated database that includes information on 3107 patients from 24 clinical studies [42]. Most of the side effects noted in controlled clinical trials of citalopram are associated with the digestive (eg, nausea) or nervous (eg, tremor) system [43]. During the 2nd and 3rd phase of the antidepressant study, it was found that of all side effects, only the incidence of nausea, dry mouth, drowsiness, trembling and sweating exceeded the corresponding rates in patients who received placebo. In addition, the prevalence of any adverse events observed while taking citalopram over the frequency of the same events associated with placebo did not exceed 10% in any of the cases. Although the adverse event profile was similar in the elderly and younger patients included in the study, their incidence was lower in the older age group [33, 44].
The safety and tolerability of citalopram in elderly patients has been confirmed in several smaller studies. The Scandinavian study, which lasted 6 weeks, involved 149 patients. Only 37% of those who received citalopram reported adverse events. Among those who took placebo, this figure was 25% [37]. In a meta-analysis of data on 325 patients (aged 19 to 94 years), long-term (8 to 296 weeks) treated with citalopram, it was found that the prevalence of side effects (which were mostly mild) is only 4% or even lower. Moreover, such side effects as increased fatigue, sleep disorders, headache and tremor were most often noted [45]. The majority of patients (53%) from the previously reviewed study did not observe any side effects at all [41]. In the same cases when they were noted, the degree of their severity in 69% was rated as mild, 20% as moderate, and only 11% as severe. Very few patients withdrew from the study due to intolerance to citalopram. In another open-label study of citalopram lasting 8 weeks, 48. 8% of 162 patients reported no side effects. And only in 24.7% of cases transient side effects were noted [33].
8% of 162 patients reported no side effects. And only in 24.7% of cases transient side effects were noted [33].
As is typical for other SSRIs, any side effects caused by citalopram are more likely to occur during the first few weeks of therapy and decrease with continued treatment [33]. Accordingly, before stopping the antidepressant, it is advisable to try to reduce its dosage.
Effects on cognitive and psychomotor functions
A double-blind, crossover study compared the psychomotor effects of citalopram, amitriptyline, and placebo in 36 healthy subjects [47]. Based on the results of a battery of electroencephalographic, psychological, subjective, and clinical tests, the researchers concluded that citalopram has little to no effect on cognitive and psychomotor functions, its sedative properties are minimal, and the main side effects are nausea, anorexia, and insomnia. On the contrary, amitriptyline had a much stronger effect on psychomotor functions.
The data presented are supported by the results of a recent randomized, double-blind, placebo-controlled, cross-over study. This study compared the effects of citalopram and the tricyclic antidepressant dothiepine on cognitive and psychomotor functions in 14 healthy volunteers [48]. Subjects were assessed with a battery of psychometric tests before and after administration of citalopram (10, 20, or 40 mg once daily), dothiepine (75 mg once daily), and placebo. Citalopram improved cognitive function (as documented by an increase in the critical threshold of the flicker-fusion test), did not affect psychomotor functions, and did not have a sedative effect. In contrast, dothiepine significantly worsened the results of most psychometric tests, including the critical threshold of the flicker-fusion test.
Newth et al. [36] in their study of citalopram in elderly depressed patients found that the antidepressant did indeed improve various aspects of cognition in a subset of dementia patients.
Safety in the treatment of depression in patients with cardiovascular disease
The presence of depression in patients with coronary heart disease (CHD) carries an additional risk of disease progression and mortality. Until now, doctors often do not prescribe antidepressants due to concerns about possible cardiotoxicity of drugs [39, 49]. Although SSRIs may be associated with cardiac arrhythmias, the prevalence of adverse cardiovascular events is very low, less than 0.0003% of cases [35].
Orthostatic hypotension is one of the most significant side effects of TCAs. One study compared the ability of citalopram and clomipramine to cause this phenomenon. While taking clomipramine (n=17), a significant decrease in systolic blood pressure (BP) was observed, which remained stable when taking citalopram (n=15) [50]. The author concluded that citalopram may be useful in the treatment of depression in elderly patients, especially those with CAD.
The experts studied more than 6500 electrocardiogram (ECG) records of 1700 patients stored in the database [20]. One-third of these patients were over 60 years of age, 277 had a previous cardiac pathology, and 97 were receiving concomitant treatment at the time of the examination or shortly before, which included medications that themselves could cause cardiac arrhythmias. ECG studies have shown that citalopram does not lead to any significant changes in the ECG, including the QT interval. The only exception is a small and clinically insignificant decrease in the number of heartbeats (by 5–9beats per minute) at the beginning of therapy in a small number of patients [20]. Many years of experience with the use of citalopram confirms the data of early studies on the absence of cardiotoxicity in the antidepressant [33].
Safety in the treatment of depression in patients with hepatic impairment
Citalopram does not cause liver disease. Initial clinical studies reported only transient and sporadic changes in laboratory parameters, and post-marketing observations did not reveal any hepatotoxicity with the considered antidepressant [33]. However, since citalopram is partially metabolized in the liver, it is recommended to reduce the dose of the drug in patients with impaired liver function.
However, since citalopram is partially metabolized in the liver, it is recommended to reduce the dose of the drug in patients with impaired liver function.
Overdose safety
Suicide occurs more frequently among the elderly than in any other age group. Moreover, the highest frequency of suicides is observed among men 85 years and older. The proportion of such people among all those who committed suicide is 23% [14, 19]. According to Yesavage [51], the problem is so serious that one can even speak of an epidemic. Since the risk of suicide is significantly increased in depressed patients, low overdose toxicity may be an important property of any antidepressant, especially one given to elderly patients. Citalopram has shown low toxicity in animal experiments, and this property is confirmed by the favorable outcome in cases where several patients overdosed on the antidepressant in question. One report indicated that the patient took 2000 mg of citalopram (a dose 100 times the usual daily dose), after which a coma developed. In the future, however, the condition did not have any serious consequences. It should be noted here that the gap between the therapeutic and toxic dose of TCA, on the contrary, is very small [33].
In the future, however, the condition did not have any serious consequences. It should be noted here that the gap between the therapeutic and toxic dose of TCA, on the contrary, is very small [33].
Interaction with other drugs
Drug interaction is an important property of antidepressants that must be taken into account when prescribing them to elderly patients, since they often take a large number of drugs at the same time, may have a reduced level of drug metabolism in the liver and increased (compared with young patients) sensitivity to psychotropic drugs [53]. In general, citalopram and sertraline appear to be the SSRIs that are least likely to have drug interactions compared to other antidepressants in this group [54]. Although the list of drugs whose metabolism is dependent on 2D6 CTC is quite large (includes new antiarrhythmics; beta-blockers; various antipsychotics, such as haloperidol and thioridazine; the modern antidepressant venlafaxine; TCAs - amitriptyline, clomipramine, desipramine, imipramine and nortriptyline; SSRIs - fluoxetine and paroxetine), citalopram only slightly inhibits this enzyme [32, 33, 54, 58]. For this reason, the concentration of the listed drugs in the blood plasma only slightly increases when they are co-administered with citalopram [33, 59–64].
For this reason, the concentration of the listed drugs in the blood plasma only slightly increases when they are co-administered with citalopram [33, 59–64].
Other studies have shown that citalopram has little or no interaction with alcohol [47]. Citalopram should not be co-administered with any RIMAOI. The outcome of a moderate overdose of moclobemide in combination with citalopram is such that it convincingly demonstrates all the possible fatality of the combination therapy of OMAI with any serotonergic drug [52].
Dosing regimen
The recommended starting dose of citalopram is 20 mg once daily, regardless of the age of the patient. Taking into account the clinical outcome of treatment and the severity of depression, the dosage in the elderly can be increased, reaching a maximum of 40 mg once a day [45, 66]. Although elderly patients are usually more sensitive to psychotropic drugs than younger patients, high doses of antidepressants, including citalopram, given for a sufficiently long period of time are necessary in order to derive the maximum benefit from psychopharmacotherapy and achieve a higher chance of recovery [1, 17, 67]. Newth et al. [36], based on the results of their own study, concluded that the effective dose of citalopram for the treatment of depression manifested in the elderly ranges from 20 to 30 mg, and in some cases it is necessary to prescribe 40 mg.
Newth et al. [36], based on the results of their own study, concluded that the effective dose of citalopram for the treatment of depression manifested in the elderly ranges from 20 to 30 mg, and in some cases it is necessary to prescribe 40 mg.
The same dosage of citalopram used to treat depression in the acute phase should also be given as maintenance therapy [17, 68]. There is evidence that maintenance therapy for 6 months after the resolution of the first episode of major depression and for 12 months after the second or third episode prevents the recurrence of mood disorders [17].
Discussion
For the elderly, an ideal antidepressant, in addition to being effective, should have minimal side effects, a safe interaction profile with other drugs and alcohol, and be taken once a day. In addition, it is desirable that this drug does not have active metabolites [51]. Citalopram meets all of these criteria. A strong serotonin reuptake inhibitor and the most selective of all SSRIs, this antidepressant has the same potency as TCAs and other SSRIs. Citalopram also has a beneficial effect on behavioral disorders in elderly patients, including agitation. Its half-life is 33 hours (slightly higher in the elderly), which allows the administration of an antidepressant once a day. Metabolites of citalopram do not affect the action of the drug itself.
Citalopram also has a beneficial effect on behavioral disorders in elderly patients, including agitation. Its half-life is 33 hours (slightly higher in the elderly), which allows the administration of an antidepressant once a day. Metabolites of citalopram do not affect the action of the drug itself.
The side effects of citalopram are usually mild and decrease in severity over several weeks of treatment. The most common side effects of an antidepressant are nausea, headache, tremors, dry mouth, and ejaculation problems. Citalopram has a low potential to interact with alcohol, as well as with most drugs, including beta-blockers, antipsychotics, lithium, TCAs. Unlike TCAs, citalopram does not cause anticholinergic side effects, orthostatic hypotension, drowsiness during the day, is not associated with cardiotoxicity, adverse effects on cognitive and psychomotor functions [37]. In overdose, citalopram is generally safe but, like other SSRIs, it should not be co-administered with RIMAOIs.