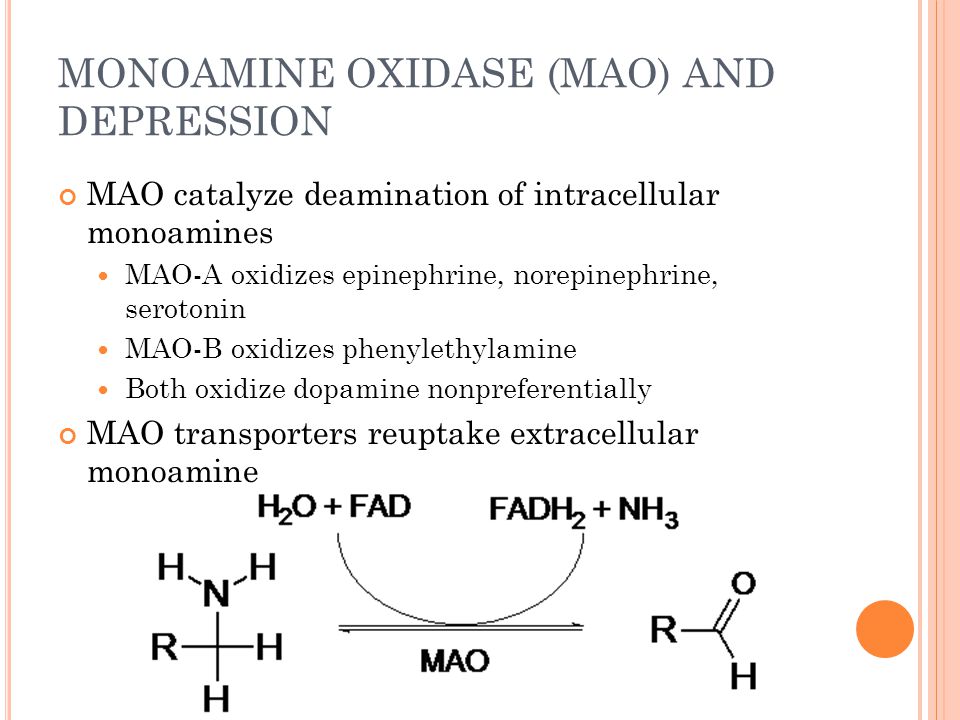
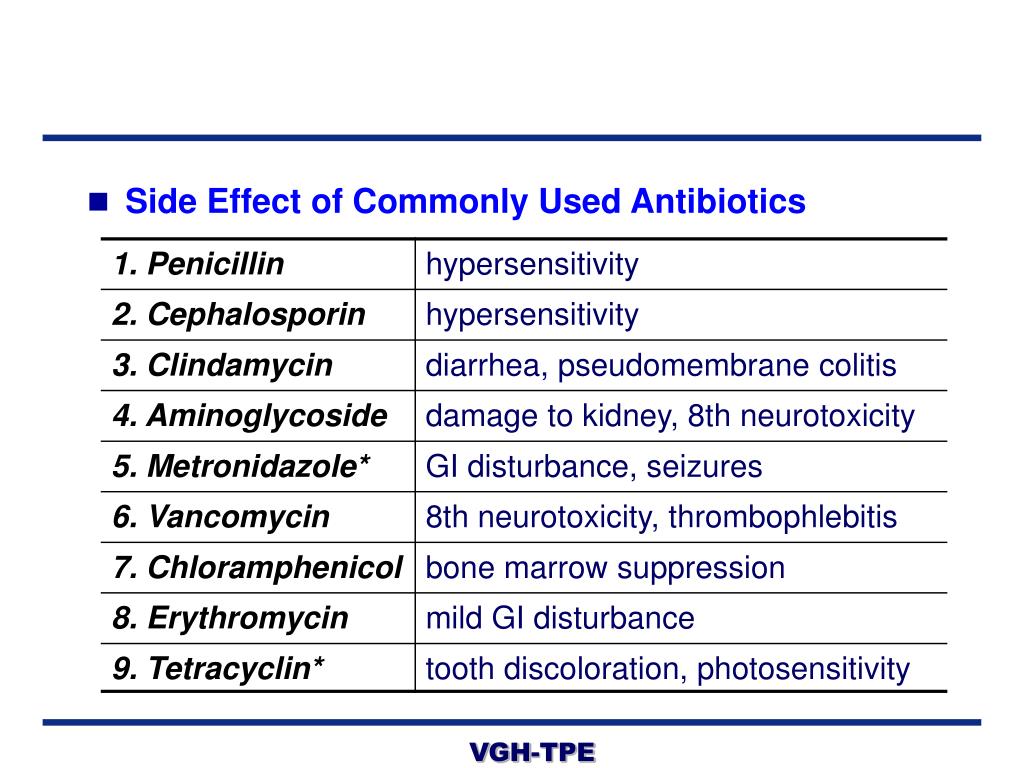
Lamotrigine common side effects
Side effects of lamotrigine - NHS
Like all medicines, lamotrigine can cause side effects, although not everyone gets them.
Most side effects of lamotrigine wear off, but it can take several weeks or months.
When you start taking lamotrigine, it's important to increase the dose slowly as this will help reduce or stop some side effects happening.
Lamotrigine can also cause some unpredictable side effects no matter what dose you take, and these can happen at any time.
Speak to your pharmacist or doctor if you're concerned about side effects.
Common side effects
These common side effects of lamotrigine may happen in more than 1 in 10 people. There are things you can do to help you cope:
HeadachesMake sure you rest and drink plenty of fluids. Do not drink too much alcohol. Ask your pharmacist to recommend a painkiller.
Talk to your doctor if your headaches last longer than a week or are severe.
Feeling drowsy, sleepy or dizzyAs your body gets used to lamotrigine, these side effects should wear off. Do not drive, ride a bike or operate machinery until you feel more alert.
If they do not go within a week or two, your doctor may reduce your dose or increase it more slowly. If that does not work, speak to your doctor. You may need to switch to a different medicine.
Aggression, or feeling irritable or agitatedTalk to your doctor.
Shaking or tremorsTalk to your doctor if this is bothering you. These symptoms can be a sign that the dose is too high for you. It may help to change your dose or take your medicine at a different time of day.
Talk to your doctor.
DiarrhoeaDrink lots of fluids, such as water or squash, to avoid dehydration. Signs of dehydration include peeing less than usual or having dark, strong-smelling pee.
Do not take any other medicines to treat diarrhoea without speaking to a pharmacist or doctor. Speak to a doctor if symptoms get worse or last longer than a week.
If you take contraceptive pills and you have severe diarrhoea for more than 24 hours, your contraception may not protect you from pregnancy. Check the pill packet to find out what to do.
Feeling or being sick (nausea or vomiting)Stick to simple meals and do not eat rich or spicy food. It might help to take your lamotrigine after a meal or snack. If you're being sick, take small, frequent sips of water or squash to avoid dehydration.
Speak to a doctor if symptoms get worse or last longer than a week.
If you take contraceptive pills and you're being sick, your contraception may not protect you from pregnancy. Check the pill packet to find out what to do.
Mild skin rashIf you get a mild rash speak to your doctor for advice as your treatment may need to be changed.
Also see our serious side effects information.
If this advice does not help and you are bothered by any of these side effects, keep taking the medicine but tell your doctor or pharmacist.
Serious side effects
Skin rashes
It's common to get a skin rash with lamotrigine. Most skin rashes are not serious.
Stevens-Johnson syndrome is a rare side effect of lamotrigine.
It causes flu-like symptoms, followed by a red or purple rash that spreads and forms blisters. The affected skin eventually dies and peels off.
The affected skin eventually dies and peels off.
It's more likely to happen in the first 8 weeks of starting lamotrigine, or when the dose is increased too quickly.
It can also happen if lamotrigine is stopped suddenly for a few days and then restarted at the same dose as before, without reducing the dose and then increasing it slowly again.
Stevens-Johnson syndrome is more common in:
- children
- people who have developed a rash before with a different epilepsy medicine
- people who are allergic to an antibiotic called trimethoprim
- people also taking a medicine called sodium valproate
To help reduce the chance of you getting a rash that could be confused with Stevens-Johnson syndrome, it's best to not try any new medicines or food during the first 3 months of treatment with lamotrigine.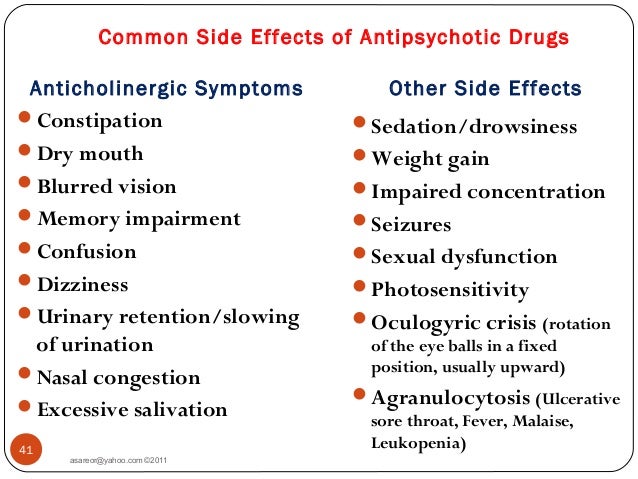
Immediate action required: Go to A&E now if:
- you get a severe rash with flushing, blisters or ulcers – these can be signs of Stevens-Johnson syndrome
Find your nearest A&E
Other serious side effects
Very few people taking lamotrigine have any serious problems as any serious reaction is quite rare.
Tell a doctor or contact 111 straight away if you have a serious side effect, including:
- worsening fits or seizures (if you take lamotrigine for epilepsy)
- unexpected bruising or bleeding, a high temperature or sore throat – these could be signs of a blood disorder
Go to 111.nhs.uk or call 111.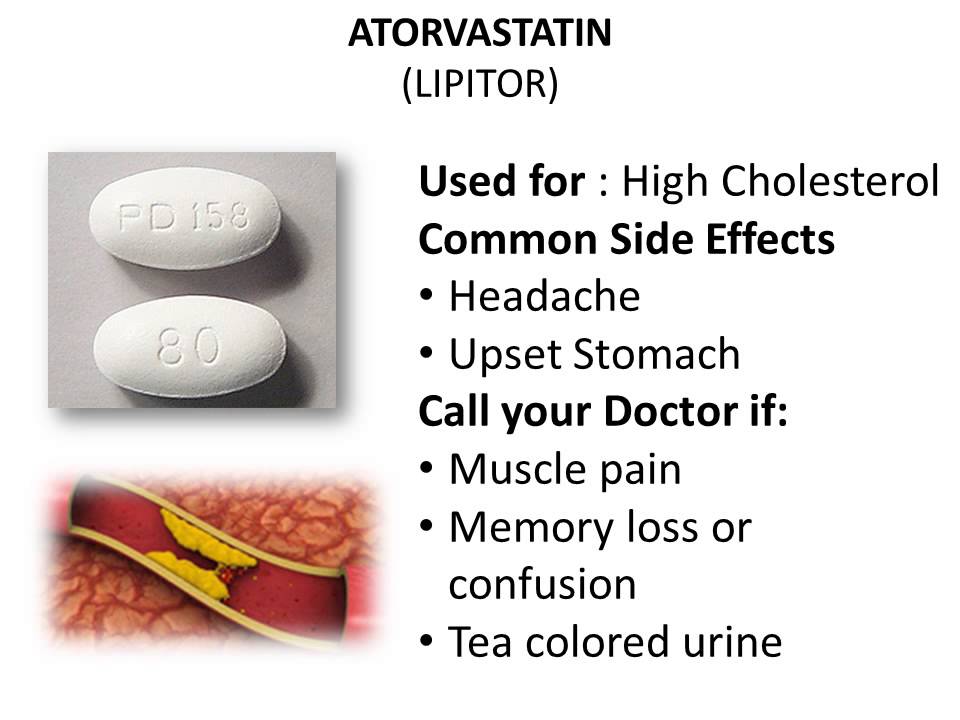
Immediate action required: Go to A&E or call 999 now if:
- you have thoughts of harming or killing yourself – a small number of people taking lamotrigine for bipolar disorder have had suicidal thoughts, and this can happen after only a few weeks of treatment
- you have a stiff neck, headaches, feel or are being sick, a high temperature and extreme sensitivity to bright light – these could be signs of meningitis
Serious allergic reaction
In rare cases, it's possible to have a serious allergic reaction (anaphylaxis) to lamotrigine.
Immediate action required: Call 999 or go to A&E now if:
- you get a skin rash that may include itchy, red, swollen, blistered or peeling skin
- you're wheezing
- you get tightness in the chest or throat
- you have trouble breathing or talking
- your mouth, face, lips, tongue or throat start swelling
You could be having a serious allergic reaction and may need immediate treatment in hospital.
Long-term side effects
Long-term treatment with lamotrigine can cause osteoporosis and osteopenia, increasing your risk of breaking a bone.
Your doctor can arrange for tests to check your bone strength.
Regular exercise and a good diet can also help keep your bones strong.
Other side effects
These are not all the side effects of lamotrigine. For a full list, see the leaflet inside your medicine packet.
Information:
You can report any suspected side effect using the Yellow Card safety scheme.
Visit Yellow Card for further information.
Page last reviewed: 20 May 2022
Next review due: 25 May 2025
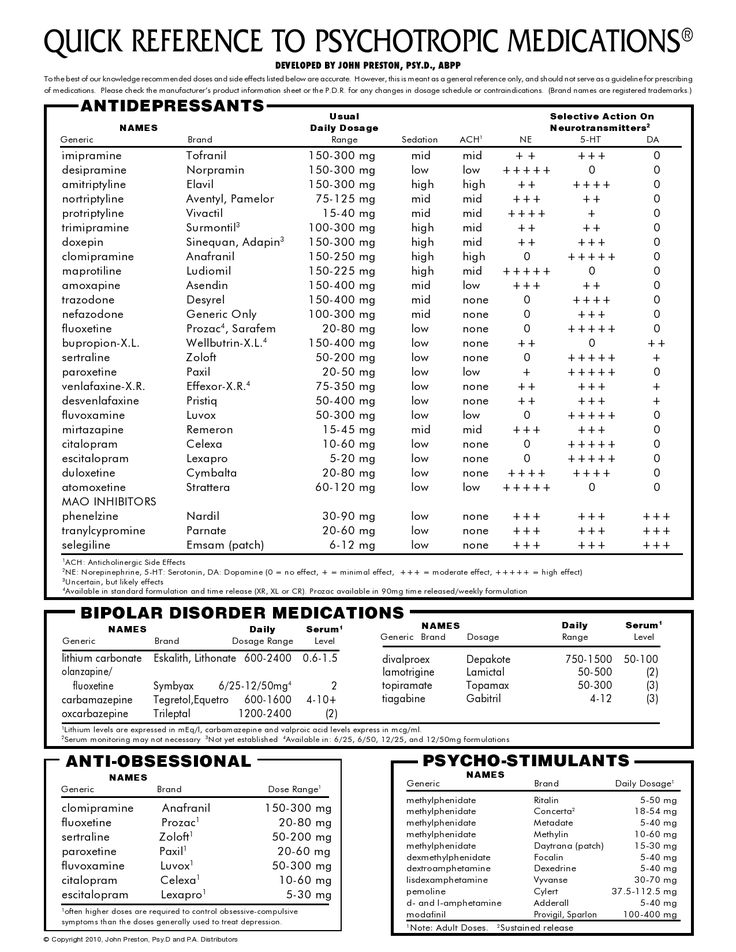
Side Effects, Dosage, Uses, and More
This drug has black box warnings. These are the most serious warnings from the Food and Drug Administration (FDA). Black box warnings alert doctors and patients about drug effects that may be dangerous.
These are the most serious warnings from the Food and Drug Administration (FDA). Black box warnings alert doctors and patients about drug effects that may be dangerous.
- Life threatening rash: This drug can cause rare but serious rashes that can be life-threatening. These rashes can occur at any time, but they’re most likely to happen within the first 2 to 8 weeks of starting this drug. Don’t increase your dosage of this drug more quickly than your doctor tells you. Your doctor may have you stop taking this drug at the first sign of rash.
- Lamotrigine oral tablet is available as brand-name drugs and as generic drugs. Brand names: Lamictal, Lamictal XR, Lamictal CD, and Lamictal ODT.
- Lamotrigine comes in four forms: immediate-release oral tablets, extended-release oral tablets, chewable oral tablets, and orally disintegrating tablets (can be dissolved on the tongue).
- Lamotrigine oral tablet is a prescription drug used to treat certain types of seizures in people with epilepsy.
 It’s also used to treat bipolar disorder.
It’s also used to treat bipolar disorder.
Lamotrigine is a prescription drug. It comes in four forms to be taken by mouth (orally): immediate-release oral tablets, extended-release oral tablets, chewable oral tablets, and orally disintegrating tablets (can be dissolved on the tongue).
Lamotrigine is available as the brand-name drugs Lamictal, Lamictal XR (extended-release), Lamictal CD (chewable), and Lamictal ODT (dissolves on the tongue).
It’s also available as generic drugs. Generic drugs usually cost less than brand-name versions. In some cases, they may not be available in every strength or form as the brand-name drugs.
Lamotrigine may be used as part of a combination therapy. This means that you may need to take it with other medications.
Why it’s used
Lamotrigine is used to treat certain types of seizures in people with epilepsy. It can be used in combination with other antiseizure medications. Or it can be used alone when switching from other antiseizure medications.
Or it can be used alone when switching from other antiseizure medications.
Lamotrigine is also used for long-term treatment of a mood disorder called bipolar disorder. With this condition, a person has extreme emotional highs and lows.
How it works
Lamotrigine belongs to a class of drugs called anticonvulsants or antiepileptic drugs (AEDs). A class of drugs is a group of medications that work in a similar way. These drugs are often used to treat similar conditions.
For people with epilepsy, this drug reduces the release of a substance in your brain known as glutamate. This action prevents the neurons in your brain from becoming too active. As a result, you may have fewer seizures.
For people with bipolar disorder, this drug may affect certain receptors in your brain that help control your mood. This could decrease the number of mood episodes you have.
Lamotrigine oral tablet may cause drowsiness. Do not drive, use heavy machinery, or do other dangerous activities until you know how this drug affects you.
Lamotrigine can also cause other side effects.
More common side effects
The more common side effects that can occur with use of lamotrigine include:
- dizziness
- drowsiness
- headache
- double vision
- blurred vision
- nausea and vomiting
- diarrhea
- stomach pain
- trouble with balance and coordination
- trouble sleeping
- back pain
- stuffy nose
- sore throat
- dry mouth
- fever
- rash
- tremor
- anxiety
If these effects are mild, they may go away within a few days or a couple of weeks. If they’re more severe or don’t go away, talk with your doctor or pharmacist.
Serious side effects
Call your doctor right away if you have serious side effects. Call 911 if your symptoms feel life threatening or if you think you’re having a medical emergency. Serious side effects and their symptoms can include the following:
- Serious skin rashes called Stevens-Johnson syndrome and toxic epidermal necrolysis.
 Symptoms can include:
Symptoms can include: - blistering or peeling of your skin
- hives
- rash
- painful sores in your mouth or around your eyes
- Multi-organ hypersensitivity, which is also called drug reaction with eosinophilia and systemic symptoms (DRESS). Symptoms can include:
- fever
- rash
- swollen lymph glands
- severe muscle pain
- frequent infections
- swelling of your face, eyes, lips, or tongue
- unusual bruising or bleeding
- weakness or tiredness
- yellowing of your skin or the white part of your eyes
- Low blood cell counts. Symptoms can include:
- tiredness
- weakness
- frequent infections or an infection that won’t go away
- unexplained bruising
- nosebleeds
- bleeding from the gums
- Changes in mood or behavior. Symptoms can include:
- thoughts about killing yourself
- attempts to harm or kill yourself
- depression or anxiety that’s new or gets worse
- restlessness
- panic attacks
- trouble sleeping
- anger
- aggressive or violent behavior
- crankiness that’s new or gets worse
- dangerous behavior or impulses
- extreme increase in activity and talking
- Aseptic meningitis (inflammation of the membrane that covers your brain and spinal cord).
 Symptoms can include:
Symptoms can include: - headache
- fever
- nausea and vomiting
- stiff neck
- rash
- being more sensitive to light than usual
- muscle pains
- chills
- confusion
- drowsiness
- Hemophagocytic lymphohistiocytosis (HLH, a life threatening immune system reaction). Symptoms can include:
- high fever, typically over 101°F
- rash
- enlarged lymph nodes
- Irregular heart rhythm. Symptoms can include:
- a fast, slow, or pounding heartbeat
- shortness of breath
- chest pain
- feeling lightheaded
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs affect each person differently, we cannot guarantee that this information includes all possible side effects. This information is not a substitute for medical advice. Always discuss possible side effects with a healthcare provider who knows your medical history.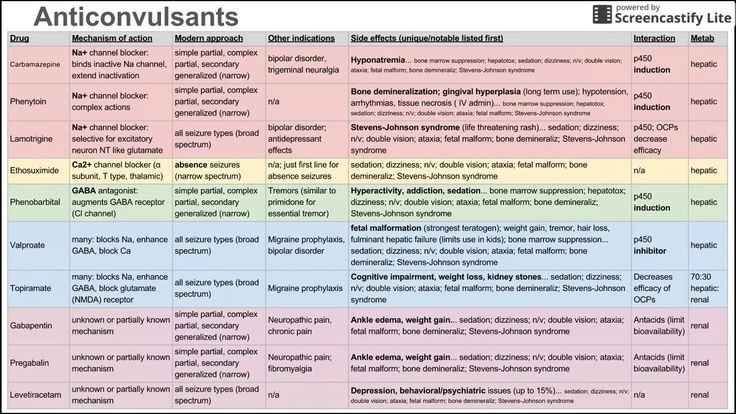
Lamotrigine oral tablet can interact with other medications, vitamins, or herbs you may be taking. An interaction is when a substance changes the way a drug works. This can be harmful or prevent the drug from working well.
To help avoid interactions, your doctor should manage all of your medications carefully. Be sure to tell your doctor about all medications, vitamins, or herbs you’re taking. To find out how this drug might interact with something else you’re taking, talk to your doctor or pharmacist.
Examples of drugs that can cause interactions with lamotrigine are listed below.
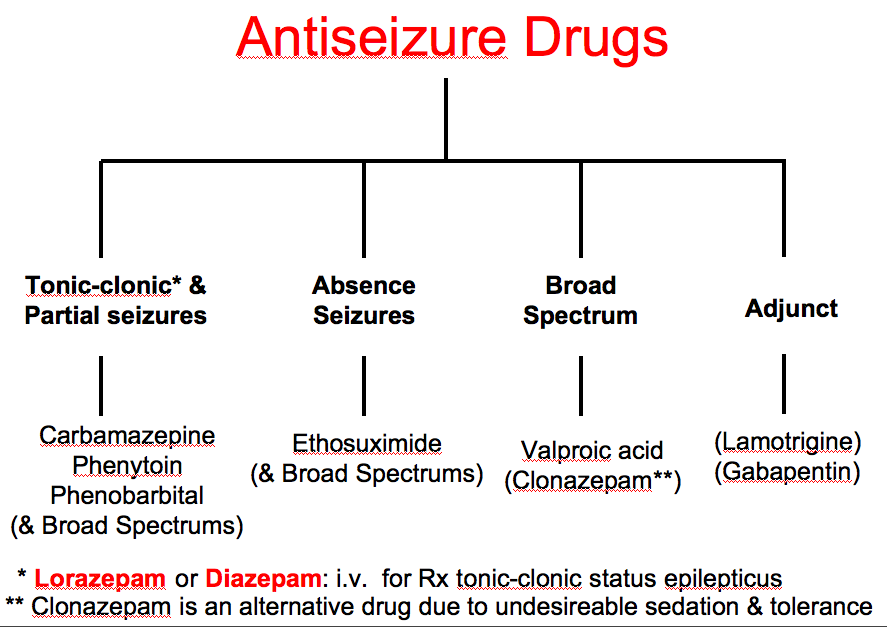
Antiseizure drugs
Taking certain other antiseizure drugs with lamotrigine can lower the level of lamotrigine in your body. This can affect how well lamotrigine works. Examples of these drugs include:
- carbamazepine
- phenobarbital
- primidone
- phenytoin
Valproate, on the other hand, can raise the level of lamotrigine in your body. This can cause increased side effects that may be dangerous.
This can cause increased side effects that may be dangerous.
Heart arrhythmia drug
Dofetilide is used to treat heart arrhythmias. When used with lamotrigine, the levels of dofetilide in your body may be increased. This may cause fatal arrhythmias.
HIV drugs
Taking lamotrigine with certain drugs used to treat HIV can lower the level of lamotrigine in your body. This can affect how well lamotrigine works. Examples of these drugs include:
- lopinavir/ritonavir
- atazanavir/ritonavir
Oral contraceptives
Taking lamotrigine with combination oral contraceptives (those that contain estrogen and progesterone) can lower the level of lamotrigine in your body. This can affect how well lamotrigine works.
Tuberculosis drug
Rifampin is used to treat tuberculosis. When used with lamotrigine, it can lower the level of lamotrigine in your body. This can affect how well lamotrigine works.
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs interact differently in each person, we cannot guarantee that this information includes all possible interactions. This information is not a substitute for medical advice. Always speak with your healthcare provider about possible interactions with all prescription drugs, vitamins, herbs and supplements, and over-the-counter drugs that you’re taking.
However, because drugs interact differently in each person, we cannot guarantee that this information includes all possible interactions. This information is not a substitute for medical advice. Always speak with your healthcare provider about possible interactions with all prescription drugs, vitamins, herbs and supplements, and over-the-counter drugs that you’re taking.
This drug comes with several warnings.
Life threatening immune system reaction
In rare cases, this drug can cause a severe immune system reaction called hemophagocytic lymphohistiocytosis (HLH). This reaction leads to severe inflammation throughout the body, and without prompt treatment, it can cause death. Common symptoms include fever, rash, and enlarged lymph nodes, liver, and spleen. They also include decreased blood cell counts, decreased liver function, and blood clotting problems.
Organ damage warningThis drug can cause serious problems to certain parts of your body. These include your liver and your blood cells.
These include your liver and your blood cells.
Suicide warning
This drug may cause thoughts of hurting yourself. Call your doctor if you notice any sudden changes in your mood, behaviors, thoughts, or feelings.
Heart disease warning
If you have had a fast heartbeat, heart failure, or other heart problems, you should not take lamotrigine. This drug may cause you to have an abnormal heartbeat, which could lead to sudden death. Symptoms include a fast, slow, or pounding heartbeat, shortness of breath, chest pain, and feeling lightheaded. Call your doctor if you experience any of these symptoms.
Allergy warning
This drug can cause a severe allergic reaction. Symptoms can include:
- rash
- trouble breathing
- swelling of your face, throat, tongue
- hives
- itching
- painful sores in your mouth
If you develop these symptoms, call 911 or go to the nearest emergency room.
Don’t take this drug again if you’ve ever had an allergic reaction to it. Taking it again could be fatal (cause death).
Taking it again could be fatal (cause death).
Warnings for people with certain health conditions
For people with liver disease: This drug is processed by your liver. If your liver isn’t working well, more of the drug may stay in your body longer. This puts you at risk for increased side effects. Your doctor may lower your dosage of this drug.
For people with kidney disease: This drug is removed from your body by your kidneys. If your kidneys aren’t working well, more of the drug may stay in your body longer. This puts you at risk for increased side effects. Your doctor may lower your dosage of this drug. If your kidney problems are severe, your doctor may stop your use of this drug or may not prescribe it at all.
For people with heart disease: This drug may lead to a fast heartbeat and cause sudden death. If you have a history of second or third-degree heart block, heart failure, abnormal heartbeat, or other heart problems, you should not use this drug.
Warnings for other groups
For pregnant women: This drug is a category C pregnancy drug. That means two things:
- Research in animals has shown adverse effects to the fetus when the mother takes the drug.
- There haven’t been enough studies done in humans to be certain how the drug might affect the fetus.
Talk with your doctor if you’re pregnant or planning to become pregnant. This drug should be used only if the potential benefit justifies the potential risk.
If you become pregnant while taking this drug, call your doctor right away.
For women who are breastfeeding: This drug is present in breast milk and may cause serious side effects in a child who is breastfed. Tell your doctor if you’re breastfeeding your child. Ask about the best way to feed your child while you’re on this medication.
If you breastfeed while taking this drug, watch your child closely. Look for symptoms such as trouble breathing, temporary episodes when breathing stops, extreme sleepiness, or poor sucking. Call your child’s doctor right away if any of these symptoms occur.
Call your child’s doctor right away if any of these symptoms occur.
For children: It isn’t known if the immediate-release version of this drug is safe and effective for treating seizures in children younger than 2 years. It also isn’t known if the extended-release version of this drug is safe and effective for children younger than 13 years.
In addition, it isn’t known if the immediate-release version of this drug is safe and effective for treating bipolar disorder in children younger than 18 years.
All possible dosages and drug forms may not be included here. Your dosage, form, and how often you take the drug will depend on:
- your age
- the condition being treated
- the severity of your condition
- other medical conditions you have
- how you react to the first dose
Drug forms and strengths
Generic: Lamotrigine
- Form: oral tablet
- Strengths: 25 mg, 50 mg, 100 mg, 150 mg, 200 mg
- Form: chewable tablet
- Strengths: 2 mg, 5 mg, 25 mg
- Form: orally disintegrating tablet (can be dissolved on the tongue)
- Strengths: 25 mg, 100 mg, 200 mg
- Form: extended-release tablet
- Strengths: 25 mg, 50 mg, 100 mg, 200 mg, 250 mg, 300 mg
Brand: Lamictal
- Form: oral tablet
- Strengths: 25 mg, 100 mg, 150 mg, 200 mg
Brand: Lamictal CD
- Form: chewable tablet
- Strengths: 2 mg, 5 mg, 25 mg
Brand: Lamictal ODT
- Form: orally disintegrating tablet (can be dissolved on the tongue)
- Strengths: 25 mg, 50 mg, 100 mg, 200 mg
Brand: Lamictal XR
- Form: extended-release tablet
- Strengths: 25 mg, 50 mg, 100 mg, 200 mg, 250 mg, 300 mg
Dosage for seizures in people with epilepsy
Adult dosage (ages 18–64 years)
Immediate-release form (tablets, chewable tablets, orally disintegrating tablets)
- TAKING with valproate:
- Weeks 1–2: Take 25 mg every other day.
- Weeks 3–4: Take 25 mg per day.
- Week 5 onward: Your doctor will increase your dose by 25–50 mg once per day every 1 to 2 weeks.
- Maintenance: Take 100–400 mg per day.
- Weeks 1–2: Take 25 mg every other day.
- NOT TAKING carbamazepine, phenytoin, phenobarbital, primidone, or valproate:
- Weeks 1–2: Take 25 mg per day.
- Weeks 3–4: Take 50 mg per day.
- Week 5 onward: Your doctor will increase your dose by 50 mg once per day every 1 to 2 weeks.
- Maintenance: Take 225–375 mg per day, in 2 divided doses.
- TAKING carbamazepine, phenytoin, phenobarbital, or primidone and NOT TAKING valproate:
- Weeks 1–2: Take 50 mg every day.
- Weeks 3–4: Take 100 mg per day, in 2 divided doses.
- Week 5 onward: Your doctor will increase your dose by 100 mg once per day every 1 to 2 weeks.
- Maintenance: Take 300–500 mg per day, in 2 divided doses.
Extended-release form (tablets)
- TAKING with valproate:
- Weeks 1–2: Take 25 mg every other day.
- Weeks 3–4: Take 25 mg per day.
- Week 5: Take 50 mg per day.
- Week 6: Take 100 mg per day.
- Week 7: Take 150 mg per day.
- Maintenance: Take 200–250 mg per day.
- NOT TAKING carbamazepine, phenytoin, phenobarbital, primidone, or valproate:
- Weeks 1–2: Take 25 mg every day.
- Weeks 3–4: Take 50 mg per day.
- Week 5: Take 100 mg per day.
- Week 6: Take 150 mg per day.
- Week 7: Take 200 mg per day.
- Maintenance: Take 300–400 mg per day.
- TAKING carbamazepine, phenytoin, phenobarbital, or primidone and NOT TAKING valproate:
- Weeks 1–2: Take 50 mg per day.

- Weeks 3–4: Take 100 mg per day.
- Week 5: Take 200 mg per day.
- Week 6: Take 300 mg per day.
- Week 7: Take 400 mg per day.
- Maintenance: Take 400–600 mg per day.
- Weeks 1–2: Take 50 mg per day.
Conversion from adjunctive therapy to monotherapy
Your doctor may choose to stop your other antiseizure medications and have you take lamotrigine by itself. This dosing will be different from what is outlined above.
Your doctor will slowly increase your dosage of lamotrigine and slowly decrease the dosages of your other antiseizure medications.
Conversion from immediate-release to extended-release (XR) lamotrigine
Your doctor can switch you directly from the immediate-release form of lamotrigine to the extended-release (XR) form. This dosing will be different from what is outlined above.
Once you switch to the XR form, your doctor will monitor you to make sure your seizures are under control. Your doctor may change your dosage based on how you respond to treatment.
Your doctor may change your dosage based on how you respond to treatment.
Child dosage (ages 13–17 years)
Immediate-release form (tablets, chewable tablets, orally disintegrating tablets)
- TAKING with valproate:
- Weeks 1–2: Take 25 mg every other day.
- Weeks 3–4: Take 25 mg per day.
- Week 5 onward: Your doctor will increase your dose by 25–50 mg once per day every 1 to 2 weeks.
- Maintenance: Take 100–400 mg per day.
- NOT TAKING carbamazepine, phenytoin, phenobarbital, primidone, or valproate:
- Weeks 1–2: Take 25 mg per day.
- Weeks 3–4: Take 50 mg per day.
- Week 5 onward: Your doctor will increase your dose by 50 mg once per day every 1 to 2 weeks.
- Maintenance: Take 225–375 mg per day, in 2 divided doses.
- TAKING carbamazepine, phenytoin, phenobarbital, or primidone and NOT TAKING valproate:
- Weeks 1–2: Take 50 mg every day.

- Weeks 3–4: Take 100 mg per day, in 2 divided doses.
- Week 5 onward: Your doctor will increase your dose by 100 mg once per day every 1 to 2 weeks.
- Maintenance: Take 300–500 mg per day, in 2 divided doses.
- Weeks 1–2: Take 50 mg every day.
Extended-release form (tablets)
- TAKING with valproate:
- Weeks 1–2: Take 25 mg every other day.
- Weeks 3–4: Take 25 mg per day.
- Week 5: Take 50 mg per day.
- Week 6: Take 100 mg per day.
- Week 7: Take 150 mg per day.
- Maintenance: Take 200–250 mg per day.
- NOT TAKING carbamazepine, phenytoin, phenobarbital, primidone, or valproate:
- Weeks 1–2: Take 25 mg every day.
- Weeks 3–4: Take 50 mg per day.
- Week 5: Take 100 mg per day.
- Week 6: Take 150 mg per day.

- Week 7: Take 200 mg per day.
- Maintenance: Take 300–400 mg per day.
- TAKING carbamazepine, phenytoin, phenobarbital, or primidone and NOT TAKING valproate:
- Weeks 1–2: Take 50 mg per day.
- Weeks 3–4: Take 100 mg per day.
- Week 5: Take 200 mg per day.
- Week 6: Take 300 mg per day.
- Week 7: Take 400 mg per day.
- Maintenance: Take 400–600 mg per day.
Conversion from adjunctive therapy to monotherapy
Your doctor may choose to stop your other antiseizure medications and have you take lamotrigine by itself. This dosing will be different from what is outlined above.
Your doctor will slowly increase your dose of lamotrigine and slowly decrease the doses of your other antiseizure medications.
Conversion from immediate-release to extended-release (XR) lamotrigine
Your doctor can switch you directly from the immediate-release form of lamotrigine to the extended-release (XR) form. This dosing will be different from what is outlined above.
This dosing will be different from what is outlined above.
Once you switch to the XR form, your doctor will monitor you to make sure your seizures are under control. Your doctor may change your dose based on how you respond to treatment.
Child dosage (ages 2–12 years)
Immediate-release form (tablets, chewable tablets, orally disintegrating tablets)
- TAKING with valproate:
- Weeks 1–2: Take 0.15 mg/kg per day, in 1–2 divided doses.
- Weeks 3–4: Take 0.3 mg/kg per day, in 1–2 divided doses.
- Week 5 onward: Your doctor will increase dose by 0.3 mg/kg per day every 1 to 2 weeks.
- Maintenance: Take 1–5 mg/kg per day, in 1–2 divided doses (maximum of 200 mg per day).
- NOT TAKING carbamazepine, phenytoin, phenobarbital, primidone, or valproate:
- Weeks 1–2: Take 0.3 mg/kg per day, in 1–2 divided doses.

- Weeks 3–4: Take 0.6 mg/kg per day, in 2 divided doses
- Week 5 onward: Your doctor will increase dose by 0.6 mg/kg per day every 1 to 2 weeks.
- Maintenance: Take 4.5–7.5 mg/kg per day, in 2 divided doses (maximum of 300 mg per day).
- Weeks 1–2: Take 0.3 mg/kg per day, in 1–2 divided doses.
- TAKING carbamazepine, phenytoin, phenobarbital, or primidone and NOT TAKING valproate:
- Weeks 1–2: Take 0.6 mg/kg per day, in 2 divided doses.
- Weeks 3–4: Take 1.2 mg/kg per day, in 2 divided doses.
- Week 5 onward: Your doctor will increase dose by 1.2 mg/kg per day every 1 to 2 weeks.
- Maintenance: Take 5–15 mg/kg per day, in 2 divided doses (maximum of 400 mg per day).
Extended-release form (tablets)
It has not been confirmed that lamotrigine is safe and effective for use in children younger than 13 years. It should not be used in these children.
Child dosage (ages 0–1 year)
Immediate-release form (tablets, chewable tablets, orally disintegrating tablets)
It has not been confirmed that these forms of lamotrigine are safe and effective for use in children younger than 2 years. They should not be used in these children.
Senior dosage (ages 65 years and older)
Older adults may process drugs more slowly. A typical adult dose may cause drug levels in your body to be higher than normal. This can be dangerous. To help avoid this, your doctor may start you on a lower dose or a different schedule.
Dosage for bipolar disorder
Adult dosage (ages 18–64 years)
Immediate-release form (tablets, chewable tablets, orally disintegrating tablets)
- TAKING with valproate:
- Weeks 1–2: Take 25 mg every other day.
- Weeks 3–4: Take 25 mg per day.
- Week 5: Take 50 mg per day.
- Week 6: Take 100 mg per day.
- Week 7: Take 100 mg per day.
- NOT TAKING carbamazepine, phenytoin, phenobarbital, primidone, or valproate:
- Weeks 1–2: Take 25 mg per day.
- Weeks 3–4: Take 50 mg per day.
- Week 5: Take 100 mg per day.
- Week 6: Take 200 mg per day.
- Week 7: Take 200 mg per day.
- TAKING carbamazepine, phenytoin, phenobarbital, or primidone and NOT TAKING valproate:
- Weeks 1–2: Take 50 mg per day.
- Weeks 3–4: Take 100 mg per day, in divided doses.
- Week 5: Take 200 mg per day, in divided doses.
- Week 6: Take 300 mg per day, in divided doses.
- Week 7: Take up to 400 mg per day, in divided doses.
Child dosage (ages 0–17 years)
Immediate-release forms (tablets, chewable tablets, orally disintegrating tablets)
It has not been confirmed that these forms of lamotrigine are safe and effective for use in children younger than 18 years for the treatment of bipolar disorder. They should not be used in children in this age range for the treatment of bipolar disorder.
They should not be used in children in this age range for the treatment of bipolar disorder.
Senior dosage (ages 65 years and older)
Older adults may process drugs more slowly. A typical adult dosage may cause drug levels in your body to be higher than normal. This can be dangerous. To help avoid this, your doctor my start you on a lower dosage or a different dosing schedule.
Special dosage considerations
- For people with liver disease: If you have moderate to severe liver problems, your doctor may lower your dosage of lamotrigine.
- For people with kidney disease: If you have kidney problems, your doctor may lower your dosage of lamotrigine. If your kidney problems are severe, talk with your doctor about whether you should use this drug.
Dosage warnings
Your starting dosage of lamotrigine should not be higher than the recommended starting dosage. Also, your dosage should not be increased too quickly.
If your dosage is too high or increased too quickly, you’re at higher risk for a serious or life threatening skin rash.
If you’re taking this drug to treat seizures and are supposed to stop taking it, your doctor will slowly lower your dosage over at least 2 weeks. If your dosage isn’t slowly lowered and tapered off, you will be at increased risk for having more seizures.
Disclaimer: Our goal is to provide you with the most relevant and current information. However, because drugs affect each person differently, we cannot guarantee that this list includes all possible dosages. This information is not a substitute for medical advice. Always speak with your doctor or pharmacist about dosages that are right for you.
Lamotrigine oral tablet is used for long-term treatment. It comes with risks if you don’t take it as prescribed.
If you stop taking the drug suddenly or don’t take it at all: If you take this drug to treat seizures, stopping the drug suddenly or not taking it at all may cause serious problems. These include an increased risk of seizures.
These include an increased risk of seizures.
They also include risk of a condition called status epilepticus (SE). With SE, short or long seizures occur for 30 minutes or more. SE is a medical emergency.
If you take this drug to treat bipolar disorder, stopping the drug suddenly or not taking it at all may cause serious problems. Your mood or behavior may get worse. You may need to be admitted to the hospital.
If you miss doses or don’t take the drug on schedule: Your medication may not work as well or may stop working completely. In order for this drug to work well, a certain amount needs to be in your body at all times.
If you take too much: You could have dangerous levels of the drug in your body. If you think you’ve taken too much of this drug, call your doctor or seek guidance from the American Association of Poison Control Centers at 1-800-222-1222 or through their online tool. But if your symptoms are severe, call 911 or go to the nearest emergency room right away.
What to do if you miss a dose: Take it as soon as you remember. If you remember just a few hours before the time for your next dose, only take one dose. Never try to catch up by taking two tablets at once. This could result in dangerous side effects.
How to tell if the drug is working: If you take this drug to treat seizures, you should have fewer seizures or less severe seizures. Be aware that you may not feel the full effect of this drug for several weeks.
If you take this drug to treat bipolar disorder, you should have fewer episodes of extreme moods. Be aware that you may not feel the full effect of this drug for several weeks.
Keep these considerations in mind if your doctor prescribes lamotrigine for you.
General
- All forms of this drug can be taken with or without food.
- Take this drug at the time(s) recommended by your doctor.
- You can cut or crush the chewable and regular oral tablets.
 You should not crush or cut the extended-release or orally disintegrating tablets.
You should not crush or cut the extended-release or orally disintegrating tablets.
Storage
- Store the oral, chewable, and extended-release tablets at room temperature at 77°F (25°C).
- Store orally disintegrating tablets at a temperature between 68°F and 77°F (20°C and 25°C).
- Keep these drugs away from light.
- Don’t store these drugs in moist or damp areas, such as bathrooms.
Refills
A prescription for this medication is refillable. You should not need a new prescription for this medication to be refilled. Your doctor will write the number of refills authorized on your prescription.
Travel
When traveling with your medication:
- Always carry your medication with you. When flying, never put it into a checked bag. Keep it in your carry-on bag.
- Don’t worry about airport X-ray machines. They can’t harm your medication.
- You may need to show airport staff the pharmacy label for your medication.
 Always carry the original prescription-labeled container with you.
Always carry the original prescription-labeled container with you. - Don’t put this medication in your car’s glove compartment or leave it in the car. Be sure to avoid doing this when the weather is very hot or very cold.
- Swallow regular and extended-release tablets whole. If you have trouble swallowing, talk to your doctor. There may be another form of this drug you can take.
- If you’re taking an orally disintegrating tablet, place it under your tongue and move it around your mouth. The tablet will quickly dissolve. It can be swallowed with or without water.
- The chewable tablets can be swallowed whole or chewed. If you chew the tablets, drink a small amount of water, or fruit juice mixed with water, to help with swallowing. The tablets can also be mixed in water, or fruit juice mixed with water. Add the tablets to 1 teaspoon of liquid (or enough to cover the tablets) in a glass or spoon. Wait at least 1 minute or until the tablets are completely dissolved.
 Then mix the solution together and drink the whole amount.
Then mix the solution together and drink the whole amount.
Clinical monitoring
Your doctor will monitor you. During your treatment with this drug, you may have tests to check for:
- Liver problems: Blood tests will help your doctor decide if it’s safe for you to start taking the drug and if you need a lower dosage.
- Kidney problems: Blood tests will help your doctor decide if it’s safe for you to start taking the drug and if you need a lower dosage.
- Serious skin reactions: Your doctor will monitor you for symptoms of a serious skin reaction. These skin reactions can be life threatening.
- Suicidal thoughts and behaviors: Your doctor will monitor you for thoughts of hurting yourself or related behaviors. Call your doctor if you notice any sudden changes in your mood, behaviors, thoughts, or feelings.
In addition, if you take this drug to treat seizures, you and your doctor will need to monitor how often you have seizures. This will help you make sure that this drug is working for you.
This will help you make sure that this drug is working for you.
And if you take this drug to treat bipolar disorder, you and your doctor will need to monitor how often you have mood episodes. This will help you make sure that this drug is working for you.
Availability
Not every pharmacy stocks this drug. When filling your prescription, be sure to call ahead to make sure your pharmacy carries it.
Prior authorization
Many insurance companies require a prior authorization for certain forms of this drug. This means your doctor will need to get approval from your insurance company before your insurance company will pay for the prescription.
There are other drugs available to treat your condition. Some may be better suited for you than others. Talk with your doctor about other drug options that may work for you.
Disclaimer: Healthline has made every effort to make certain that all information is factually correct, comprehensive, and up to date.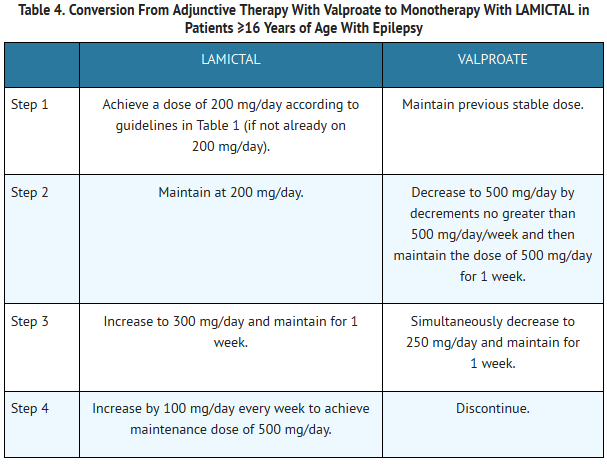 However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or other healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or other healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
| 💊 The composition of the drug Lamotrigine ✅ The use of the drug Lamotrigine Keep for yourself Search for analogues Interaction Description of the active ingredients of the preparation Lamotrigine (Lamotrigine) The scientific information provided is general and cannot be used to make decisions. Update date: 2020.04.27 Marketing authorization holder:ATOLL, OOO (Russia)
Manufactured:OZON, OOO (Russia) ATX code: N03AX09 (lamotrigine) Active substance: lamotrigine (lamotrigine) nine0006 Rec.INN WHO registered Dosage form
Release form, packaging and composition drug LamotrigineTablets white or white with a yellowish tint, round, flat-cylindrical, notched on one side and chamfered on both sides.
Excipients : lactose monohydrate (milk sugar) - 167.8 mg, microcrystalline cellulose - 76 mg, povidone K-25 - 19 mg, sodium carboxymethyl starch - 11.4 mg, magnesium stearate - 38 colloidal dioxide - 2 mg. 10 pcs. - cellular contour packings (1) - packs of cardboard. Clinical and pharmacological group: Antiepileptic drug Pharmacotherapeutic group: Antiepileptic Pharmacological action Anticonvulsant. The mechanism of action is associated with the effect on voltage-dependent sodium channels of the presynaptic membrane. This leads to a decrease in the release of mediators into the synaptic cleft, primarily glutamate, an excitatory amino acid that plays an important role in the formation of epileptic discharges in the brain. PharmacokineticsAfter oral administration, lamotrigine is rapidly and completely absorbed from the gastrointestinal tract. C max in plasma is reached after about 2.5 hours. Plasma protein binding is 55%. It undergoes intensive metabolism with the formation of the main metabolite N-glucuronide. T 1/2 in adults is an average of 29 hours Excreted by the kidneys mainly as a metabolite; about 8% of the active substance is excreted unchanged. T 1/2 less in children than in adults. Indications of the active substances of the drug Lamotrigine nine0021Partial and generalized tonic-clonic seizures (more often in cases of resistance to treatment with other anticonvulsants). Open list of ICD-10 codes
Dosing regimen The route of administration and dosing regimen of a particular drug depends on its form of release and other factors. When taken orally for adults and children over 12 years of age, the initial single dose is 25-50 mg, maintenance doses are 100-200 mg / day. In rare cases, doses of 500-700 mg / day may be required. For children aged 2 to 12 years, the initial dose is 0.2-2 mg/kg/day, the maintenance dose is 1-15 mg/kg/day. The maximum daily dose of for children aged 2 to 12 years, depending on the treatment regimen used, is 200-400 mg. The frequency of administration, the intervals between doses with increasing doses depend on the treatment regimen used, the patient's response to the treatment. Side effectsFrom the side of the central nervous system: headache, dizziness, drowsiness, sleep disturbances, fatigue, aggressiveness, confusion. From the digestive system: nausea, abnormal liver function. From the side of the hematopoietic system: leukopenia, thrombocytopenia. nine0006 Allergic reactions: skin rash (usually maculopapular), angioedema, Stevens-Johnson syndrome, toxic epidermal necrolysis, lymphadenopathy. Contraindications for useSevere hepatic impairment, hypersensitivity to lamotrigine. Use in pregnancy and lactationClinical data on the safety of the use of lamotrigine during pregnancy and lactation are insufficient. nine0006 When deciding on the need for use during pregnancy, the expected benefit of therapy for the mother and the potential risk to the fetus should be compared. Preliminary data show that lamotrigine passes into breast milk at a concentration of 40-45% of the plasma concentration. A small number of infants whose mothers received lamotrigine did not experience side effects. Use in hepatic impairmentContraindicated in severe hepatic impairment. nine0006 Use in impaired renal function Use with caution in patients with renal insufficiency. Use in childrenDo not use lamotrigine in children under 2 years of age. Elderly useDo not use lamotrigine in elderly patients. Special instructionsUse with caution in patients with renal insufficiency. Lamotrigine should not be used in elderly patients. nine0006 If severe allergic skin reactions occur, lamotrigine should be discontinued. Abrupt withdrawal of lamotrigine may increase the manifestations of epilepsy, so it is necessary to gradually stop treatment, reducing the dose over 2 weeks. When used simultaneously with carbamazepine, dizziness, diplopia, ataxia, visual disturbances, nausea are possible. These phenomena, as a rule, disappear with a decrease in the dose of carbamazepine. Do not use lamotrigine in children under 2 years of age. nine0006 Influence on the ability to drive vehicles and mechanisms During the treatment period, a slowdown in the speed of psychomotor reactions is observed. Drug interactionsSimultaneous use with anticonvulsants - inducers of liver metabolism (including phenytoin, carbamazepine, phenobarbital, primidone) accelerates the metabolism of lamotrigine. With the simultaneous use of lamotrigine and carbamazepine or phenytoin, a decrease in T 1/2 lamotrigine. There have been reports of dizziness, ataxia, diplopia, blurred vision, and nausea in patients taking carbamazepine after starting treatment with lamotrigine. Due to the inhibition of microsomal liver enzymes under the influence of sodium valproate, with simultaneous use slows down the metabolism of lamotrigine, increases T 1/2 lamotrigine. Keep | nine0050
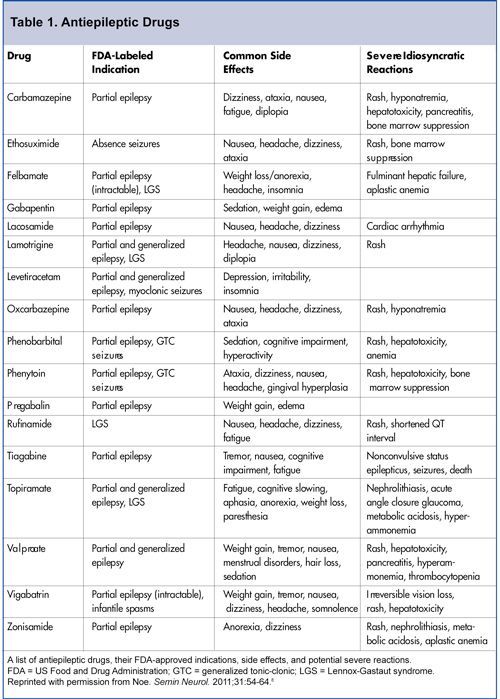
| Treatment of epilepsy involves long-term use of antiepileptic drugs (AEDs), but sometimes, after some time after the start of treatment, the previously prescribed AED has to be replaced with another one. Distinguish between acute and chronic toxic effects that antiepileptic drugs have [1]. Among them, non-specific dose-dependent, specific pharmacodynamic effects for a given drug and rare, but potentially life-threatening idiosyncratic reactions are distinguished. The first two types of reactions can usually be diagnosed and prevented in a timely manner. The latter type of reactions, as a rule, is unpredictable and develops very quickly, which practically does not allow diagnostics at the preclinical stage [2-5]. The main serious side effects of this type include hepatitis, pancreatitis, bone marrow suppression, and exfoliative dermatitis. These effects, if not recognized in time, can lead to the death of the patient [6], and the reaction does not depend on the dose of the drug and is unique in each case [1]. nine0006 Potentially life-threatening idiosyncratic reactions are listed in Table 1. Idiosyncratic reactions usually develop within the first 6 months after starting the drug. Their frequency is approximately 1:30,000 - 1:50,000. This risk is higher for the development of Stevens-Johnson syndrome for lamotrigine - about 1:1000 [7], aplastic anemia for felbamate - about 1:5000 [8] and liver damage in young children with polytherapy with the inclusion of valproic acid preparations - about 1:500 [9]. AED hypersensitivity syndrome is observed at 2-8 weeks of aromatic AED treatment. AED hypersensitivity syndrome is observed at 2-8 weeks of aromatic AED treatment. Symptoms are rash, fever, and eosinophilia. Lymphadenopathy and potentially life-threatening necrotic changes in the liver may also occur [10]. More severe reactions include exfoliative dermatitis, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis [11], which is presumably based on the pharmacogenetic features of biotransformation [12]. When prescribing any AED that is at risk of developing Stevens-Johnson and Lyell syndrome or anticonvulsant hypersensitivity syndrome [13], the patient (parents or caregivers) should be warned of the risk of rash and the need for daily skin examination. The patient should be instructed on what action to take if a rash is detected. The doctor should plan in advance a possible reserve AED, which has two properties: no risk of developing skin complications and the possibility of prescribing a therapeutic dose from the first day (valproate, levetiracetam). nine0006 Currently, there are no reliable ways to prevent idiosyncratic reactions. Currently, there are no reliable ways to prevent idiosyncratic reactions. Taking into account the proposed mechanism of immediate type hypersensitivity [14], it is proposed to use a mast cell degranulation test before prescribing a drug or a lymphocyte transformation reaction, taking into account the alleged role of epoxide hydrolase deficiency (a component of the cytochrome oxidase system) in the pathogenesis of these conditions [12,15]. Neurotoxic effects in the form of drowsiness, dizziness, affective changes (depression, agitation), impaired memory, attention, performance when using AED are quite common [1,16]. Tracking these effects is especially necessary in the early stages of treatment, because. over time, the patient forgets his "normal" state - the one that was before the appointment of the drug. A few months after the appointment of AED, the patient himself and his relatives begin to perceive the "new" state as "normal". And only after discontinuation of the drug, patients often notice that the world around them began to be perceived brighter, the level of cognitive functioning increased, and working capacity increased. Thus, the so-called. the phenomenon of "adaptation to the drug" does not mean that the neurotoxic effect disappears over time, the patient simply forgets how he felt before treatment [4]. In the early stages of therapy, it is especially important to ask the patient about his state of health, to try to accurately identify neurotoxic symptoms (for example, drowsiness with carbamazepine or slow thinking with topiramate). When taking AEDs, more pronounced neurotoxic effects may also be observed: tremor, diplopia, sometimes dysarthria, ataxia [1,16]. Neurotoxic effects can be pharmacokinetic and pharmacodynamic [17]. Therefore, when neurotoxic effects appear, it is necessary to determine the level of the drug in the blood to exclude the pharmacokinetic mechanism of their occurrence. Protocols for monitoring the presence of chronic specific adverse effects of AEDs are currently not published [4]. Most authors recommend conducting a preliminary examination, during which a risk group is identified in relation to the development of undesirable effects of the use of AEDs [4,17]. This group consists of patients with changes in biochemical parameters, neurodegenerative diseases, concomitant somatic diseases, and a history of severe idiosyncratic reactions [17]. The observation plan for the timely detection of the occurrence of undesirable effects depends on the specific clinical situation (type and doses of the drug used, risk factors). Table 2 lists the major serious adverse effects of AEDs. Below are descriptions of the main side effects of AEDs presented on the Russian pharmaceutical market. Barbiturates commonly cause behavioral problems in children (hyperactivity, irritability) and drowsiness in adults. Attention disturbances, thought disorders, and depression may be both dose-dependent and independent of plasma concentrations of the drug in sensitive patients [18]. Dose-dependent neurotoxic effects include nystagmus, ataxia, dyskinesia, and disruption of sleep patterns. nine0006 Idiosyncratic reactions include allergic dermatitis, Stevens-Johnson syndrome, serum sickness (serum sickness, the term is used in the English literature and for non-protein drugs) and liver damage, agranulocytosis and aplastic anemia [5]. Exacerbation of porphyria may be noted [17]. Being inducers of microsomal enzymes, barbiturates alter the metabolism of vitamin D, which leads to the development of osteoporosis, especially in childhood. Being inducers of microsomal enzymes, barbiturates alter the metabolism of vitamin D, which leads to the development of osteoporosis, especially in childhood and immobilized patients [17]. In addition, barbiturates interfere with absorption and accelerate the metabolism of vitamin K. In this regard, newborns from mothers receiving barbiturates need to be given vitamin K to prevent hemorrhagic disease of the newborn. The pregnant woman should receive vitamin K during the last month of pregnancy, at the beginning of the birth process, and the child should be given vitamin K immediately after delivery [17]. nine0006
Barbiturates interfere with the absorption and accelerate the metabolism of vitamin K, therefore, newborns from mothers receiving barbiturates need to be given vitamin K to prevent hemorrhagic disease of the newborn. With chronic use of barbiturates, changes in the connective tissue can be observed with the development of coarsening of facial features, Dupuytren's contracture, plantar fibromatosis and stiffness of the shoulder joint [17]. Phenytoin is characterized by non-linear pharmacokinetics and a narrow therapeutic range, which often leads to the development of dose-dependent adverse effects (nystagmus, ataxia, dyskinesia, and cognitive impairment) not only at the beginning of treatment due to drug accumulation [5]. Children may sometimes be irritable or hyperactive. The combination of anorexia, vomiting and weight loss are characteristic of phenytoin intoxication in childhood [17]. There are also a number of dose-independent effects. With prolonged use of the drug, there is a coarsening of facial features, a change in the texture and color of the hair, gum hyperplasia, the appearance of acne, the development of osteoporosis and lymphadenopathy. Folic acid deficiency with phenytoin can lead to megaloblastic anemia and transient encephalopathy. nine0006 Folic acid deficiency with phenytoin can lead to megaloblastic anemia and transient encephalopathy. Long-term elevated plasma levels of phenytoin can lead to cerebellar atrophy. Potentially life-threatening idiosyncratic reactions include allergic skin reactions, hepatopathy, serum sickness-like reactions, and aplastic anemia [5]. The development of a systemic drug reaction in the form of lupus erythematosus has also been described [17]. nine0006 Ethosuximide in a high single dose causes vomiting, abdominal pain. These phenomena can be reduced by taking the drug during meals and by reducing the single dose by increasing the frequency of taking the drug. Rare idiosyncratic effects include skin rashes, leukopenia, pancytopenia, and aplastic anemia [10]. Neurotoxic effects include headaches, which can be very intense, as well as drowsiness, agitation, aggressiveness, depression, and memory impairment. Mental disorders are associated with the phenomenon of forced normalization of the EEG. In children, the development of a systemic drug reaction similar to lupus has also been described [17]. Carbamazepine causes a moderate dose-dependent neurotoxic effect. At the beginning of treatment, transient symptoms may occur in the form of nausea, drowsiness, dizziness, ataxia and blurred speech, which regress within a week without changing the dose of the drug. Diplopia, tremor and headache occur with increasing plasma concentrations of the drug and can serve as a clinical marker for dose titration [16]. Occasionally, nausea, vomiting, diarrhea, and abdominal pain may occur. Hyponatremia is common but rarely reaches a clinically significant level. These dose-dependent effects are less pronounced when using prolonged forms of the drug [17]. nine0006 Skin rashes occur in 5-8% of patients, but exfoliative dermatitis and bullous-type reactions (eg, Stevens-Johnson syndrome) are rare [16]. Hematological changes occur quite often - leukopenia is noted in 10-12% of patients. Leukopenia occurs in 10-12% of patients receiving carbamazepines. However, fatal reactions such as aplastic anemia are rare, with a mortality rate of about 1.1:500,000 treated with carbamazepine per year. Despite the fact that this opinion is not shared by all authors [4,17], we consider it appropriate to conduct hematological control to exclude the progressive nature of leukopenia. As suggested by the Canadian Association of Child Neurologists [19], we perform a clinical blood test with platelet count before starting treatment. Then, taking into account the life span of cytopopulations, it is advisable to carry out hematological control after 5-6, 14-16 and 30 days from the start of taking the drug (or increasing its dose). In the future, in the first year of treatment, hematological control is carried out 1 time in 3 months, with a longer use of the drug - 1 time in 6 months. Taking into account the possible hepatotoxic effect [16], we suggest monitoring the activity of liver enzymes within 1 month from the start of taking the drug, then once every 6 months and according to indications. Valproate can cause gastrointestinal symptoms - nausea, vomiting, abdominal pain, diarrhea, which in most patients disappear when prolonged forms of the drug are prescribed and taken with food. Tremor during stress and at rest depends on the dose of the drug and, possibly, the age of the patient, because. in children, this symptom is less common. An increase in body weight due to an increase in appetite is observed in 20-54% of patients, which in some cases requires discontinuation of the drug. Hair loss is another common unwanted effect. The effect is transient. Hair becomes brittle, and newly growing hair changes its structure, becoming more curly. The use of multivitamins containing folic acid and zinc can reduce this undesirable phenomenon. There is evidence of the possibility of developing polycystic ovaries during treatment with valproate. There are data on the possibility of developing polycystic ovaries in the treatment with valproate [21]. In this regard, it is recommended that girls and women undergo pelvic ultrasound with the determination of the volume of the ovaries before the start of treatment, followed by control once a year. Idiosyncratic reactions include acute encephalopathy and coma, which may occur early in treatment. In a laboratory study, pronounced acidosis and increased excretion of organic acids are revealed. It is assumed that this complication occurs in patients with a previously compensated defect in mitochondrial beta-oxidases [22,23]. Allergic skin reactions are extremely rare, but can be quite severe [24]. Acute hemorrhagic pancreatitis can be fatal, and therefore in patients with abdominal pain during treatment with valproate, it is necessary to determine the level of lipase and amylase [17]. nine0006 The most severe idiosyncratic reaction associated with this drug is fulminant irreversible liver damage. Most often, this complication occurs against the background of polytherapy in children under the age of 2 years, with severe epilepsy, mental retardation, and signs of organic brain damage [9]. Routine laboratory tests do not allow to identify a risk group and catch the initial signs of liver damage: in many patients with advanced liver damage, laboratory data remained within the normal range, while in other patients there is often a change in liver tests without evidence of clinically significant liver damage [23] . Gabapentin causes mild neurotoxic manifestations, but severe behavioral disorders (aggression, hyperactivity) may occur in mentally retarded children. Lamotrigine may cause neurotoxic effects such as fatigue, drowsiness and confusion [28]. A feature of the beginning of treatment with lamotrigine is the need for a slow dose titration. This is due to the risk of developing skin rashes in the first 8 weeks of therapy, in rare cases, up to the development of Stevens-Johnson and Lyell syndromes. nine0006 The risk of rash when lamotrigine 5-10% is used with valproate increases to 14%. The risk of rash when lamotrigine 5-10% is used with valproate increases to 14%. Predictors of the development of a rash are: a rash against the background of another AED in history; children under 13 years of age; combined use with valproate [29]. Currently, slow titration of the dose of lamotrigine is considered mandatory. Pregabalin may cause neurotoxic effects (dizziness, ataxia, drowsiness) and weight gain [30]. Levetiracetam may cause headache, irritability, drowsiness and anorexia [31]. Significant behavioral disorders and psychoses have been described in childhood [32]. Topiramate causes dose-dependent neurotoxic effects (ataxia, dizziness, paresthesia, impaired concentration, impaired thinking, fatigue, drowsiness, confusion, psychosis) [33]. Slow titration of the drug reduces the risk of these effects. Other serious complications in the treatment of this drug are weight loss, skin rashes. Acute secondary glaucoma may also occur, and therefore the patient should be warned about the need to immediately contact a specialist in case of pain in the eyes and visual impairment [34]. Oxcarbazepine is a derivative of carbamazepine, which is rapidly converted to the active metabolite 10,11-dihydro-10-hydroxycarbamazepine, due to which its toxic effects are less pronounced compared to carbamazepine. Dose-dependent neurotoxic effects (dizziness, drowsiness, fatigue) may occur. Hyponatremia can be quite pronounced. Skin rashes, Stevens-Johnson syndrome, and exfoliative dermatitis are rare with oxcarbazepine [36]. Thus, the appointment of any AED carries the risk of side effects. It is necessary to identify groups of patients where this risk may be increased. The patient (or his parents, guardian) must be warned about the occurrence of possible undesirable effects. During the treatment of epilepsy, it is necessary to carry out clinical and laboratory monitoring according to an individually drawn up observation plan for the earliest possible detection of possible side effects, especially in the first six months of treatment with the drug. If signs of undesirable effects appear, the patient should contact the attending physician to decide on the correction of antiepileptic therapy. nine0041 | |||||||||
| Osteoporosis | x | x | 1 550 9005 CB ESC, ethosuximide; GBP, gabapentin; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; PB, phenobarbital; TPM - Topamax VPA - Valproic Acid. |
 decisions about the possibility of using a particular drug. nine0006
decisions about the possibility of using a particular drug. nine0006  100 mg: 10, 20, 25, 30, 40, 50, 60, 75, 90, 100, 125, 150, 250, 300 or 500 pcs.
100 mg: 10, 20, 25, 30, 40, 50, 60, 75, 90, 100, 125, 150, 250, 300 or 500 pcs.  - cellular contour packings (2) - packs of cardboard.
- cellular contour packings (2) - packs of cardboard. 
 The optimal dosage regimen is determined by the doctor. Compliance of the dosage form of a particular drug with indications for use and dosing regimen should be strictly observed. nine0048
The optimal dosage regimen is determined by the doctor. Compliance of the dosage form of a particular drug with indications for use and dosing regimen should be strictly observed. nine0048 

 This must be taken into account by persons engaged in potentially hazardous activities that require increased attention and quick psychomotor reactions.
This must be taken into account by persons engaged in potentially hazardous activities that require increased attention and quick psychomotor reactions.  One of the important reasons for the forced replacement of the drug is its side effects.
One of the important reasons for the forced replacement of the drug is its side effects. 
 nine0006
nine0006  nine0006
nine0006  Comparison of the results of simple non-labor-intensive tests to assess the level of attention, working capacity, short-term memory and psychomotor tests before treatment and in the first months after its start reveals the appearance of neurotoxic effects of the drug. We suggest conducting such testing before prescribing the drug, and then, in the absence of complaints, after 1 month from the start of taking the drug and then 1 time in 3 months in the first year of treatment and 1 time in 6 months thereafter. In children, it is advisable not to compare individual indicators during treatment in "raw" scores, but to conduct a centile assessment of test performance, because standards change with age. nine0006
Comparison of the results of simple non-labor-intensive tests to assess the level of attention, working capacity, short-term memory and psychomotor tests before treatment and in the first months after its start reveals the appearance of neurotoxic effects of the drug. We suggest conducting such testing before prescribing the drug, and then, in the absence of complaints, after 1 month from the start of taking the drug and then 1 time in 3 months in the first year of treatment and 1 time in 6 months thereafter. In children, it is advisable not to compare individual indicators during treatment in "raw" scores, but to conduct a centile assessment of test performance, because standards change with age. nine0006  With a high content of the drug in the blood, it is enough to reduce its dose, which will eliminate the side effect of the treatment. However, the presence of a dose-independent neurotoxic (pharmacodynamic) effect, as a rule, requires discontinuation of the drug. nine0006
With a high content of the drug in the blood, it is enough to reduce its dose, which will eliminate the side effect of the treatment. However, the presence of a dose-independent neurotoxic (pharmacodynamic) effect, as a rule, requires discontinuation of the drug. nine0006  nine0006
nine0006  nine0006
nine0006 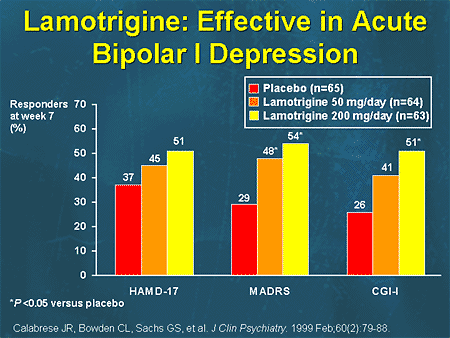

 nine0006
nine0006 
 Since the drug can lead to the development of atrioventricular blockade [16], it is desirable to monitor the ECG (1 month after the start of the drug or increasing its dose, in the future - 1 time in 6 months). nine0006
Since the drug can lead to the development of atrioventricular blockade [16], it is desirable to monitor the ECG (1 month after the start of the drug or increasing its dose, in the future - 1 time in 6 months). nine0006  The development of thrombocytopenia, which usually does not reach a clinically significant level, depends on the dose of the drug and requires a dose reduction, and in the presence of clinical symptoms (appearance of petechiae, ecchymosis) - and its withdrawal [17, 20]. For timely diagnosis of thrombocytopenia, we determine the number of platelets after 14-16 and 30 days from the start of taking the drug (or increasing its dose). In the future, in the first year of treatment, control is carried out 1 time in 3 months, with a longer use of the drug - 1 time in 6 months. nine0006
The development of thrombocytopenia, which usually does not reach a clinically significant level, depends on the dose of the drug and requires a dose reduction, and in the presence of clinical symptoms (appearance of petechiae, ecchymosis) - and its withdrawal [17, 20]. For timely diagnosis of thrombocytopenia, we determine the number of platelets after 14-16 and 30 days from the start of taking the drug (or increasing its dose). In the future, in the first year of treatment, control is carried out 1 time in 3 months, with a longer use of the drug - 1 time in 6 months. nine0006 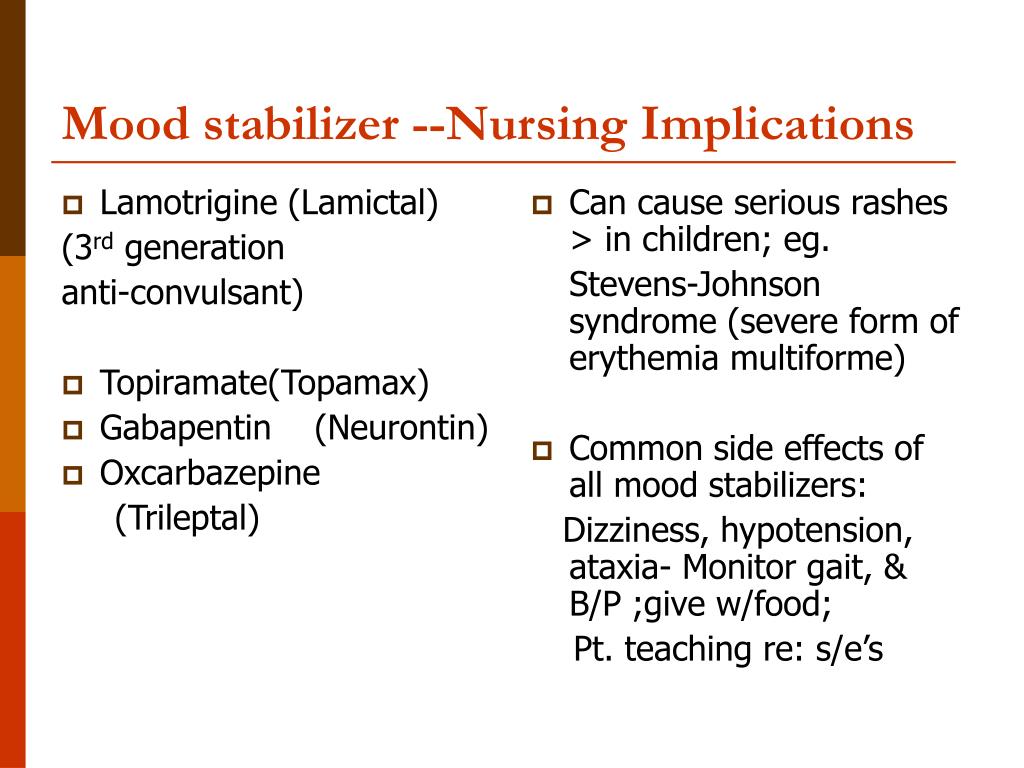
 The combination of nausea, vomiting and anorexia was observed in 82% of cases of fulminant hepatopathy, and drowsiness, decreased level of consciousness and coma - in 40% [9]. Most often, this complication develops in the first 6 months of treatment, but can also be observed within 2 years after taking valproate [9]. Specific disorders associated with an increased risk of developing acute hepatopathy include: urea cycle disorders, organic aciduria, multiple carboxylase deficiencies, mitochondrial or respiratory chain dysfunction, cytochrome aa3 deficiency in muscle tissue, pyruvate carboxylase deficiency, and insufficiency of the hepatic pyruvate dehydrogenase complex [25 ], gangliosidosis type 2 GM1, spinocerebellar degeneration, Friedreich's ataxia, Lafort's disease, Alper's disease, MERRF syndrome [26]. nine0006
The combination of nausea, vomiting and anorexia was observed in 82% of cases of fulminant hepatopathy, and drowsiness, decreased level of consciousness and coma - in 40% [9]. Most often, this complication develops in the first 6 months of treatment, but can also be observed within 2 years after taking valproate [9]. Specific disorders associated with an increased risk of developing acute hepatopathy include: urea cycle disorders, organic aciduria, multiple carboxylase deficiencies, mitochondrial or respiratory chain dysfunction, cytochrome aa3 deficiency in muscle tissue, pyruvate carboxylase deficiency, and insufficiency of the hepatic pyruvate dehydrogenase complex [25 ], gangliosidosis type 2 GM1, spinocerebellar degeneration, Friedreich's ataxia, Lafort's disease, Alper's disease, MERRF syndrome [26]. nine0006  In addition, there may be an increase in body weight and peripheral edema with a normal content of protein and albumin in the blood plasma [27].
In addition, there may be an increase in body weight and peripheral edema with a normal content of protein and albumin in the blood plasma [27].  Strict adherence to this rule reduces the risk of developing severe, life-threatening rashes without reducing the overall risk of developing benign rashes. nine0006
Strict adherence to this rule reduces the risk of developing severe, life-threatening rashes without reducing the overall risk of developing benign rashes. nine0006  Another serious adverse effect associated with carbonic anhydrase inhibition is nephrolithiasis, which occurs in 1% of patients receiving topiramate [21], which requires monitoring of urine tests and periodic renal ultrasound for the detection of calculi. Increased fluid intake can reduce the risk of this complication. Children may develop hypohidrosis and hyperthermia; which is especially important in hot weather and during outdoor games. Parents need to be informed about this, because. a child with this complication needs additional cooling due to impaired heat transfer by convection [35]. nine0006
Another serious adverse effect associated with carbonic anhydrase inhibition is nephrolithiasis, which occurs in 1% of patients receiving topiramate [21], which requires monitoring of urine tests and periodic renal ultrasound for the detection of calculi. Increased fluid intake can reduce the risk of this complication. Children may develop hypohidrosis and hyperthermia; which is especially important in hot weather and during outdoor games. Parents need to be informed about this, because. a child with this complication needs additional cooling due to impaired heat transfer by convection [35]. nine0006